Since the TGA last reviewed montelukast in 2018, medical researchers have published over 40 peer-reviewed papers (listed below in order of publication) confirming its association with neuropsychiatric side effects. That is more papers than were published in the previous 20 years, and they have made critical findings.
More common than previously thought
Melbourne’s Royal Children’s Hospital recently updated its clinical guidelines on montelukast for Pre-school and school aged children to state: “1 in 6 children may develop side effects with this medication including agitation, sleep disturbance and altered mood. If this occurs, cease medication to see if symptoms resolve.”
This reflects findings in the medical research literature which put the prevalence of neuropsychiatric events after taking montelukast as high as 5.7 per cent (Lafuente et al, 2021), 5.9 per cent (Erdam et al, 2023), 16 per cent (Ernst and Ernst, 2017), over 30 per cent (Al Shamrani et al, 2022) and 62 per cent (Yilmaz Bayer et al, 2020). Dresden Glockler-Lauf et al (2019) found asthmatic children who visited hospital for a neuropsychiatric event were almost twice as likely to have taken montelukast in the year before it.
Proposed mechanisms
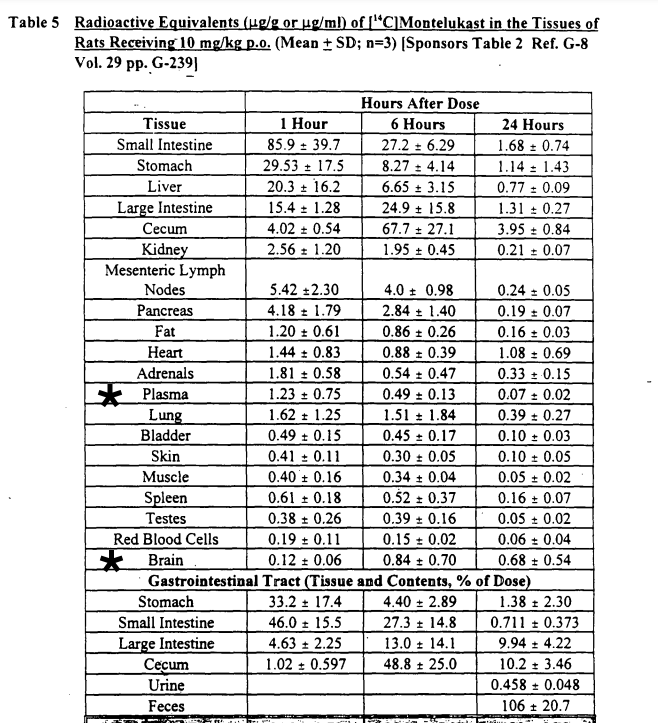
Mechanisms by which side effects occur are suggested by Marques et al (2022a, 2022b), Rostevanov et al (2022), Umetsu et al (2021), Tseng et al (2020), Eriksson et al (2018) Marschallinger et al (2015) and page 14 of this 2019 US FDA statement. In this 1998 US FDA Pharmocology review of Singulair, Merck data showing Brain/Plasma ratio in rats one hour after taking 10mg/kg montelukast is 10 per cent (0.12/1.23). After 24 hours it is 971 per cent (0.68/0.07). While the concentration of montelukast decreased in all other tissues after 24 hours, in brain tissue it increased 467 per cent (p. 14).
Adverse event database associations
In large databases of adverse event reports, montelukast is strongly associated with aggression, anxiety, suicidal ideation and depression – the adverse events most often reported to the TGA. For example,
- In the Netherlands and WHO databases, aggression in children was 29.77 times more likely while depression was reported 6.93 times more frequently (Haarman et al, 2017).
- This closely matched an analysis of the US FDA database which found suicidal ideation 21.5 times more likely and depression 8.2 times more likely to be reported (Umetsu et al, 2021).
- In a large US database, anxiety was reported 1.21 times more often (Paljarvi et al, 2022)
- Another US FDA database study found it associated 10.35 times more often than other drugs with any neuropsychiatric adverse event (Bian et al, 2021).
- In the French and WHO databases, montelukast was 12.18 times more likely to induce tics (Touafchia et al, 2020)
Longer time to onset
When it reviwed montelukast in 2018, the TGA claimed time to onset of adverse events was within 14 days, which would make it easy for parents and doctors to identify them. However, more recent research shows they can occur months to years later. This, combined with the lack of a logical connection between mental health issues and a respiratory medication, means patients and caregivers can fail to connect them to montelukast and thus continue to take the very medication causing their distress.
- Chung et al (2023) show that risk of depression relapse after taking montelukast increases with time.
- Erdam et al (2023) saw increasing events up to 3 months and listed the short time frame as a limitation of their study.
- Paljarvi et al (2022) found increased odds of adverse events within one year despite specifically excluding the first 14 days after prescription from analysis.
- Sholz et al (2019) report a case study of effects emerging after four months.
- Jordan et al (2023) looked at events within 90 days of medication.
- Bian et al (2021) found a median time to onset of symptoms of 31 days, with a range up to 306 days.
- Dresden Glockler-Lauf et al (2019) found twice the odds of events within a year of prescription
- Perona et al (2015) found onset times ranging from days to years.
- Callero-Viera et al (2012) list four cases where onset ranged from 3 weeks to 2 months
- Gadde et al (2010) found onset in 20 patients ranged from a few days to 8 years, with a mean of 14 months and a median of 7 months
- Explaining US FDA warnings about montelukast in a collaboration with Medscape, Dr Erica Torjusen stated, “we have reports following initiation of montelukast and after chronic use.”
Long-term effects
As an asthma preventer, montelukast is designed for long-term use, but there are no studies on its long term effects. Many children whose parents have shared their experiences in the 19,000 strong Facebook group Montelukast (Singulair) Side Effects Support and Discussion have taken this drug for over five years and suffered devastating side effects. While most have improved after coming off the drug, some have suffered permanent damaged from it.
Withdrawals
Els and Webb (2022) describe an Australian case in which side effects emerged upon withdrawal of montelukast. However, there is little knowledge of this among health professionals and no guidance on how to safely withdraw from montelukast. Doctors and patients in the US are warned in information leaflets that neuropsychiatric events can occur on discontinuation of montelukast, but Australian doctors and patients are not (see Why inferior warnings?).
Sign and share the petition to insist Australian consumers are adequately warned about the potential mental health side effects of this drug.
Neuropsychiatric adverse reactions of leukotriene receptor antagonist, antihistamine and inhaled corticosteroid: A real-world analysis of the Food and Drug Administration (FDA) Adverse Event Reporting
Bian S, Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B, Wang L, Yin J
Preprint from Authorea Preprints, 30 Jan 2024
https://europepmc.org/article/ppr/ppr795171
There are limited real-world studies about the differences of leukotriene receptor antagonist (LTRA), antihistamine and inhaled corticosteroid (ICS) associated neuropsychiatric events. We aimed in this study to summarize the clinical characteristics of drug associated neuropsychiatric events and compare the differences between different drug categories. Methods Disproportionality analysis and Bayesian analysis were used in data mining to screen the suspected neuropsychiatric events with LTRA, antihistamine and ICS based on the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) from January 2004 to September 2020. Results A total of 9475 neuropsychiatric events were identified. Neuropsychiatric events related to LTRA, antihistamine and ICS were 5201 (54.89%), 3226 (34.05%), and 1048 (11.06%), respectively. LTRA related neuropsychiatric events were more common in patients aged from 4 to 6 years old (18.66%), while antihistamine and ICS related neuropsychiatric events were more common in patients with 18 to 44 years old (29.92%) and older than 65 years old (30.60%), respectively. Montelukast was considered to have the tightest relationship to neuropsychiatric events, followed by the first generation of antihistamine. Most neuropsychiatric symptoms occurred within the first 10 days after drug initiation. Death rate due to neuropsychiatric events of antihistamine was significantly higher than LTRA and ICS (p<0.001). Conclusions LTRA associated neuropsychiatric events was most frequent in 4 to 6-year old children and most cases occurred with the first 10 days after drug initiation. Fatality rate due to antihistamine associated neuropsychiatric events was higher than LTRA and ICS.
Drug-induced psychiatric disorders: A pharmacovigilance update
François Montastruc, Tanguy Taillefer de Laportaliere
Therapies, 31 October 2023
https://www.sciencedirect.com/science/article/abs/pii/S0040595723001701?via%3Dihub
This article confirms montelukast’s association with psychiatric disorders and argues awareness raising is critical to avoiding its damaging consequences.
Phenotype and endotype based treatment of preschool wheeze
Sormeh Salehian, Louise Fleming, Sejal Saglani & Adnan Custovic
Expert Review of Respiratory Medicine, 24 October 2023
https://www.tandfonline.com/doi/full/10.1080/17476348.2023.2271832
The LTRA montelukast has been widely prescribed in pre-schoolers, although its significant side effect profile is increasingly recognized and makes this a less popular choice for clinicians. Efficacy in PSW is also inconsistent, with clinical trials demonstrating little benefit for regular LRTA. The Wheeze And Intermittent Treatment (WAIT) trial further demonstrated no improvement as compared to placebo for the previously popular regime of intermittent montelukast.
Prescribing cascades in ambulatory care: A structured synthesis of evidence
Faiza Shahid, Ann Doherty, Emma Wallace, Sven Schmiedl, G. Caleb Alexander, Tobias Dreischulte
Pharmacotherapy 25 September 2023
https://pubmed.ncbi.nlm.nih.gov/37743815/
This study found fair evidence that montelukast induced depression leading to the prescription of anti-depressants.
The Risk of Neuropsychiatric Adverse Events With Use of Leukotriene Receptor Antagonists in Patients With Asthma: Analysis of Korea’s National Health Insurance Sharing Service Database
Jung-Hyun Kim, Hyesung Lee, Dongyeon Jeong, Ji-Hyang Lee, Hyouk-Soo Kwon, Woo-Jung Song, You Sook Cho, Ye-Jee Kim, Yong-Wook Shin, Tae-Bum Kim
Journal of Allergy and Clinical Immunology: In Practice, 1 September 2023 https://www.sciencedirect.com/science/article/abs/pii/S2213219823009601
Methods: This retrospective population-based study analyzed the National Health Insurance claims records of the entire Korean population between 2008 and 2015. We compared the risk of neuropsychiatric adverse events among patients with asthma using inhaled corticosteroids and/or long-acting β2-agonists with montelukast or pranlukast and those not using leukotriene receptor antagonists (control group).
Results: There was no increased risk of the composite outcome of all measured neuropsychiatric adverse events in patients with asthma who were prescribed montelukast or pranlukast compared with those who were not. However, montelukast use was associated with an increased risk of hallucinations (inverse probability treatment weighting hazard ratio, 1.45; 95% CI, 1.07-1.96) and attention problems (inverse probability treatment weighting hazard ratio, 1.24; 95% CI, 1.01-1.52). Significant negative hazards for disorientation, anxiety, stress reactions, and somatic symptoms were observed in the montelukast group. When grouped by sex, the risk of hallucinations and attention problems was higher in men prescribed montelukast compared with the controls.
Conclusions: We did not observe an increase in all neuropsychiatric adverse events in the leukotriene receptor antagonist-treated group; however, an increased risk of hallucinations and attention problems was observed in those taking montelukast, regardless of the medication administration period.
Study on the risk signal mining related to montelukast in pediatric patients based on the US FDA Adverse Event Reporting System Database
Liu Yanhui, Ruan Wenyi, Chen Huiying, Mei Kangkang, Cai Heping.
Adverse Drug Reactions Journal. August 2023, 25(8): 469-474 https://doi.org/10.3760/cma.j.cn114015-20230227-00121
AE reports of children with montelukast as the primary suspect drug from the first quarter of 2004 to the third quarter of 2022 were collected by searching the US FDA Adverse Event Reporting System database (FAERS). AEs were standardized and classified according to the preferred terms (PT) and system organ class (SOC) of Medical Dictionary for Regulatory Activities 23.0. Proportional reporting odds ratio (PRR) method was used to mine the AE risk signals of montelukast. An AE with reports ≥3, PRR≥2, and χ2>4 was defined as a positive signal, which were analyzed using descriptive method.
Results A total of 5-179 AE reports were included in the analysis, involving 1-295 PTs, and 233 positive PTs were obtained by PRR method. The top 10 PTs in AE reports were aggressive behavior, anxiety, suicidal ideation, abnormal behavior, depression, anger, nightmares, insomnia, crying loudly and night terrors. Except crying loudly, all of them were adverse reactions recorded in the label. The top 10 PTs in signal intensity were sensory overload, arrhythmia, separation anxiety disorder, loneliness phobia, dust allergy, Mille-Fisher syndrome, eosinophilic granuloma complicated with polyangitis, personality disorder in children, night terrors and decreased platelet adhesion. Among them, abnormal heart rate, Mille-Fisher syndrome and decreased platelet adhesion were not recorded in the label. A total of 59 of the 233 positive PTs were not recorded in the label, involving 10 SOCs. The top 5 SOCs were social environment, mental illness, injury, poisoning and surgical complications, general conditions and administration site, and respiratory, thoracic and mediastinal diseases.
Conclusion The main AEs of pediatric patients receiving montelukast treatment in the US FAERS are aggressive behavior, anxiety, depression, insomnia, night terrors, etc., all of which are adverse reactions recorded in the label; adverse reactions not recorded in the drug label include abnormal heart rate, Miller-Fisher syndrome and decreased platelet adhesion.
The Association between Leukotriene-Modifying agents Use and Depression in Adults: A Population-based Analysis of the NHANES from 2007 to 2016
Jingchao Yan, Hong Sun, Xiu Xin, Taomin Huang, and Jianwen Shen, Fudan University Eye Ear Nose and Throat Hospital
June 23, 2023
“Introduction Post-market monitoring has revealed an association between the use of leukotriene-modifying agents (LTRAs) and an increased occurrence of neuropsychiatric events. However, the results of observational studies have been inconclusive. Objective To assess the potential correlation between LTRAs exposure and depression in US outpatient adults. Method This population-based cross-sectional study used data from U.S. adults aged 20 to 59 years from the National Health and Nutrition Examination Survey (NHANES) 2007-2016 cycle. The Patient Health Questionnaire-9 was used to assess depression. Multivariable regression was used to evaluate the association between LTRAs exposure and depression. Results Among the 9,539 participants (mean age 40.4 years; 56.2% male), 602 (6.3%) were classified as having depression. LTRAs exposure was associated with a higher prevalence of depression (16.7% [50] vs. 6.0% [552]). In the multivariable logistic regression model LTRAs exposure was associated with depression (odds ratio [OR], 1.85; 95% confidence interval [CI], 1.22~2.83). An association between LTRAs exposure and depression was found in sensitivity analyses that conducted multivariable linear regression with the PHQ-9 score as a continuous variable (β, 0.86; 95% CI, 0.39~1.33), regardless of the PHQ-9 cut-off of 5 or 10, and the multivariable logistic regression results showed that LTRAs use increased the risk of depression (OR = 1.51 [95% CI, 1.12~2.05]; OR = 1.85 [95% CI, 1.22~2.83]). Conclusion Long-term LTRAs exposure is positively associated with depression in the adult outpatient population in the US. Therefore, the risk for depression in patients receiving long-term LTRAs treatment should be considered.”
Basic clinical management of Preschool wheeze
Andrew Bush, Imperial College Business School, June 9, 2023
“Pharmacotherapy: leukotriene receptor antagonists Montelukast is popular and widely prescribed, but the evidence base is weak and the side-effect profile unfavourable. The theoretical basis is sound, cysteinyl leukotrienes are released during viral infections and are pro-inflammatory; but they just do not work for the majority. Respiratory viral infections cause elevations in cysteinyl leukotrienes, and treatment with intermittent or continuous montelukast has been suggested. However, recent trials are discouraging (Table 4). The two largest recent trials, recruiting over 3000 children, have failed to show benefit for either intermittent or continuous montelukast. Anecdotally a few pre-school wheezers respond to montelukast, but most do not. A therapeutic trial may be considered, but unless there is clear benefit it should be discontinued. Parents should be warned about the behavioural side-effects of montelukast which have a prevalence of around 20% and can be very distressing. Hence, for most pre-school wheezers, montelukast is not useful.”
Alice in wonderland: A rare side effect of montelukast
Edward Rojas, Robert Stansbury, Mouhannad Azzouz, Sunil Sharma, Christopher Pham, Steven Coutras, Heather Clawges
Sleep, Volume 46, Issue Supplement_1, May 2023, Page A452
https://academic.oup.com/sleep/article/46/Supplement_1/A452/7182423?login=false
“Alice in wonderland syndrome (AIWS), also known as Todd’s syndrome, is a neuropsychological condition that causes a set of symptoms with alteration of body image. These distortions include appearing smaller(micropsia) or larger(macropsia) or appearing to be closer or farther than they are. The exact cause of AIWS is currently unknown. Some associations include, migraine, temporal lobe epilepsy, brain tumors, psychoactive drugs or Epstein-Barr-virus. The treatment for this disease primarily aims at treating the underlying reason as to why the patient had the event.
Report of case(s)
7-year-old boy presents to sleep clinic for nighttime hallucinations. Nightly episodes described as body parts that seem abnormally large or small and people talking very fast. Events lasted 15 minutes. Patient had full recall and developed anxiety when falling asleep. Medications at presentation: vitamins, melatonin, Montelukast. Montelukast was discontinued. Referred to neurology for video EEG, which was negative for frontal lobe seizures. Due to restless sleep and snoring, sleep study and ferritin was ordered. He was found to have mild OSA with an AHI of 2.2 and a ferritin level of 28. The patient was not considered a surgical candidate for his mild OSA; therefore, watchful waiting and re-initiation of montelukast proceeded with initiation of oral iron. At sleep clinic follow up, family noted improvement of hallucinations for 2-3 months and then restart, with worsening. Upon medication review, Montelukast was the inciting factor to the presence and resolution of hallucinations. In this patient the montelukast seemed to be the triggering factor of AIWS. It has been suggested that the inhibition of leukotriene receptors in the brain could be responsible for this drug reaction. No other obvious triggers, or results of work up were able to better explain his resolution once he discontinued montelukast. This just further promotes close pharmacologic vigilance in the pediatric population.”
Prescribed Drugs and Self-Directed Violence: A Descriptive Study in the Spanish Pharmacovigilance Database
Avedillo-Salas, A.; Pueyo-Val, J.; Fanlo-Villacampa, A.; Navarro-Pemán, C.; Lanuza-Giménez, F.J.; Ioakeim-Skoufa, I.; Vicente-Romero, J.
Pharmaceuticals 2023, 16, 772
https://www.mdpi.com/1424-8247/16/5/772
“A descriptive, longitudinal and retrospective study of spontaneous reports of adverse drug reactions corresponding to self-directed violence was recorded in the Spanish Pharmacovigilance Database (FEDRA®) from 1984 to 31 March 2021. A total of 710 cases were reported in the study period. There were reports of montelukast, hydroxychloroquine, isotretinoin, methylphenidate, infliximab, natalizumab, ribavirin and efavirenz, which were less known to be involved in self-directed violence. This study shows that self-directed violence is a rare adverse drug reaction, and can be related to the use of some medicines. It is important for healthcare professionals to consider this risk in their clinical praxis, implementing person-centred approaches…an association was found between violence and montelukast, a leukotriene receptor antagonist used for the treatment of bronchial asthma and allergy relief, whose adverse reactions include suicidal ideation, self-injurious behavior, agitation, aggressiveness, anxiety and irritability… most cases of violence associated with montelukast occurred in children under 17 years old, mainly male…health professionals should be aware of this association in order to avoid or minimize its consequences. In addition, studies should focus on children and adolescents, as these are particularly sensitive population groups to whom medicines are prescribed that are clearly related to violent behavior. Potential research directions to address gaps in the knowledge about this relationship include studying the psychostimulants methylphenidate and atomoxetine, psychoanaleptics, psycholeptics and montelukast.”
Alice in Wonderland Syndrome
Nagham Al-Zubidi, MD, Pranessh Ravi, MS, M. Tariq Bhatti, MD
American Academy of Opthamology, EyeWiki, 28 April 2023
https://eyewiki.aao.org/Alice_in_Wonderland_Syndrome
“Alice in wonderland syndrome (AIWS) is a disorder of visual perception. It was inculcated into medical literature by Lipmann in 1952, where he described it as an impairment of time, sense and body image[1]. Symptoms are similar to those perceived by the character in the book (Alice in wonderland) by Lewis Caroll. AIWS is a rare neuro-ophthalmological entity with varied causes. No large epidemiological data has been published yet. The possible and common causes are listed below.”
| Medications | montelukast, dextromethorphan, topiramate, risperidone |
Pediatric Asthma: Where Has Montelukast Gone?
Maglione, M., Giannattasio, A., Pascarella, A., Tipo, V.
Applied Sciences, 24 March 2023
https://www.mdpi.com/2076-3417/13/7/4146
“Despite being traditionally considered as safe, with well-described anti-inflammatory and bronchoprotective activities, montelukast may determine adverse events that are mild in most cases. However, the mechanisms and clinical aspects of montelukast-related adverse reactions represent one of the main issues the literature has focused on in the last decade. Many reports have particularly highlighted the occurrence of neuropsychiatric effects ranging from nightmares and sleep disorders to hallucinations, aggressiveness, anxiety, and suicidal ideation. Such manifestations are more common in 4- to 6-year-old children and typically occur within the first ten days after first administration. A detailed characterization of the adverse drug reactions following montelukast therapy was recently provided by a systematic review analyzing 13 studies and almost 7000 treated patients, which confirmed hyperactivity, irritability, anxiety, and sleep disorders as the most widely reported psychiatric manifestations. The increasing awareness of these adverse reactions, their higher frequency in children rather than adults, and the documented negative impact on the patients’ quality of life led the FDA to strengthen the existing warnings in 2020 by requiring montelukast to have a boxed warning about serious mental health side effects.
The observation of neuropsychiatric events not only during treatment, but also occasionally after montelukast discontinuation, and the report of cases with persisting symptoms after drug withdrawal, has stimulated researchers toward the identification of the involved mechanisms. A recent study analyzing montelukast metabolic pathways both in vitro and in mouse models has stressed the role of a metabolite, a montelukast-glutathione conjugate, that might be responsible for the decreased glutathione levels in the brain tissue, with a reduction in its protective effect against oxidative stress. Furthermore, montelukast administration in mice has been shown to determine a hypothalamic–pituitary–adrenal axis dysregulation, ultimately entailing altered levels of neurotransmitters, which might induce the observed neuropsychiatric disorders [64]. Although not validated in an experimental model, some drug–gene interactions have also been hypothesized. A wide analysis of 1144 genes interacting with montelukast recently showed that some of them are related to mood disorders. Genes encoding neuropeptide precursors such as hypocretin, affecting depression or serotonin receptors and associated with schizophrenia and suicidality, might be involved in the pathogenesis of neuropsychiatric manifestations.”
Psychiatric adverse effects of montelukast – a nationwide cohort study
Jordan, A., Lindhardt Toennesen, L., Eklof, J., Sivapalan, P., Meteran, H., Bonnelykke, K., Suppli Ulrik, C., Staehr Jensen, J.
The Journal of Allergy and Clinical Immunology: In Practice, March 20 2023
https://www.jaci-inpractice.org/article/S2213-2198(23)00294-5/fulltext
“Objective: To assess if montelukast exposure in adults with asthma is associated with onset of neuropsychiatric adverse events using data from the Danish nationwide health registers.
Methods: Individuals ≥18 years with either ≥1 prescription redemption of inhaled corticosteroids (ICS) or with at least one hospital contact with asthma as the main diagnosis between January 1, 2011 and December 31, 2018 were included. Montelukast exposure was assessed as a time-dependent variable. The two outcomes of interest were: use of neuropsychiatric medicine including antidepressants, antipsychotics, anxiolytics, lithium and medication used for attention-deficit/hyperactivity disorder (outcome 1), and hospital contacts with a neuropsychiatric diagnosis (outcome 2), within 90 days of exposure to montelukast.
Results: Initiation of montelukast was significantly associated with outcome 1: use of neuropsychiatric medicine (HR (95% CI) = 1.14 (1.08-1.20, p <0.0001). In the assessment of outcome 2: hospital contacts with a neuropsychiatric diagnosis, a significant risk associated with montelukast initiation was found only in the youngest age groups (HR (95% CI) = 1.28 (1.12-1.47), p<0.001 and 1.16 (1.02-1.31), p<0.05, for age group 18–29 and 30–44, respectively). Age-stratified analyses showed that the risk of both outcomes increased with decreasing age, with the highest risk seen in patients aged 18-29.
Conclusion: Among younger individuals, montelukast use was significantly associated with an increased risk of neuropsychiatric events such as use of neuropsychiatric medicine and hospital treatment. Clinicians should increase awareness of such adverse effects when prescribing montelukast.”
Adverse drug reactions affecting treatment adherence in first-line treatment of asthma: An observational study
Semiha Bahceci Erdem, Hikmet Tekin Nacaroglu, Demet Can
Allergologia et Immunopathologia, 2023 Mar 1;51(2):11-16.
https://pubmed.ncbi.nlm.nih.gov/36916083/
“Objective: This study aims to investigate the frequency of drug discontinuation due to adverse drug reactions (ADRs) that affect adherence to treatment in children with asthma or asthma and allergic rhinitis using LTRA or ICS as monotherapy. Methods: The subjects aged 4-18 years with asthma or asthma and allergic rhinitis and using montelukast or ICS as monotherapy were included in the study. They were evaluated in terms of ADRs affecting adherence to treatment in the first and third months of treatment. Results: A total of 468 cases, 356 of whom received montelukast monotherapy and 112 of whom received ICS treatment, with a mean age of 9.10 ± 3.08 (4-17) years, were included in the study. Males constituted 65.6% of the total cases (n = 307). In the first month of follow-up of the cases, it was observed that 4.8% (n = 17) of the patients in the montelukast group could not continue the treatment due to ADR. It was determined that the drug discontinuation rate in the montelukast group in the first month was significantly higher than in the ICS group (P = 0.016), and the risk of drug discontinuation due to ADR in the montelukast group was 1.333 (95% CI, 1.26-1.40) times higher. Conclusions: As a result, it was observed that the drug was discontinued due to ADR at a higher rate in children with asthma who received montelukast monotherapy compared to those who received ICS monotherapy.”
“In the first 3 months of the treatment, the rate of drug discontinuation due to ADR was 5.9% in the montelukast group… Almost all of the ADRs in the montelukast group were neuropsychiatric in nature. Adverse effects reported in the montelukast group were hallucinations, night fears and sleep disturbances, suicidality, hyperactivity, aggressive offensive behaviors, destructivity, nausea, and vomiting. In the cases with nausea and vomiting, symptoms appeared in the first week of treatment, while neuropsychiatric ADRs were generally observed after 2–3 weeks of the treatment. Within a mean of 2.6 ± 1.45 (0–7) days following the discontinuation of treatment, it was observed that the symptoms improved within 2 days following the discontinuation of the drug in seven patients with hallucinations, and within 7 days in the patient with suicidality.
All clinicians who follow up and treat children with asthma should be aware of the relationship between montelukast and neuropsychiatric ADRs. Clinicians should consider the benefits and risks before prescribing montelukast. Although most of the neuropsychiatric ADRs that are frequently observed in the pediatric population improved clinically following the discontinuation of montelukast, studies must be conducted to determine whether they cause long-term psychiatric problems or not and their long-term effects on the quality of life of patients.
Limitations of the Study 1. Comprised a short period of time, that is, the first 3 months of treatment.
Montelukast and risk for antidepressant treatment failure
Haemy Chung, Kaitlin Hanken, Alicia K. Gerke, Brian C. Lund
Journal of Psychosomatic Research, Volume 164, 2023
https://www.sciencedirect.com/science/article/pii/S0022399922003609
“Depression relapse was significantly more likely for patients receiving montelukast over the first three months following initiation (aRR = 1.17; 95% CI: 1.00, 1.35). Post-hoc examination of hazard curves suggested the greatest relapse potential during the first month of therapy, where relapse risk within 45 days of index was significantly higher among montelukast recipients for both primary (RR = 1.50; 95% CI: 1.16, 1.93) and secondary (RR = 1.27; 95% CI: 1.05, 1.55) outcome measures. While these findings cannot be interpreted as definitive evidence, they suggest montelukast may be associated with increased risk, approximately 17%, for depression relapse within three months of initiation, and 50% within 45 days.”
Montelukast Increased IL-25, IL-33, and TSLP via Epigenetic Regulation in Airway Epithelial Cells
Tsai ML, Tsai MK, Tsai YG, Lin YC, Hsu YL, Chen YT, Lin YC, Hung CH.
International Journal of Molecular Science. 2023 Jan 8;24(2):1227
https://pubmed.ncbi.nlm.nih.gov/36674744/fulltext
“The expression of IL-25, IL-33, and TSLP was increased under LTRAs treatment and suppressed by inhaled corticosteroid cotreatment. Montelukast-induced IL-25, IL-33, and TSLP expression were mediated by the mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways and regulated by histone H3 acetylation and H3K36 and H3K79 trimethylation. LTRAs alone might increase inflammation and exacerbate asthma by inducing the production of IL-25, IL-33, and TSLP; therefore, LTRA monotherapy may not be an appropriate therapeutic option for asthma.” “Conclusions: In conclusion, through the NF-κB and MAPK pathways and histone modification, LTRAs increased IL25, IL33, and TSLP mRNA expression in lung and bronchial epithelial cells, which might provide support for the decreased effectiveness of LTRAs in asthma therapy. Therefore, LTRAs may be more effective when combined with ICS administration, and LTRA monotherapy may not be a good option for asthma.”
The mechanisms underlying montelukast’s neuropsychiatric effects – new insights from a combined metabolic and multiomics approach.
**** THIS IS A SEMINAL ARTICLE ****
Cátia F. Marques, M. Matilde Marques, Gonçalo C. Justino
Life Sciences, Volume 310, 2022
“Montelukast (MTK) is an antagonist of the cysteinyl leukotrienes receptor 1 widely used to manage asthma symptoms among adults and children. However, it has been associated with an increasing number of neuropsychiatric adverse drug reactions (ADRs), particularly among children, including depression, sleep disturbance, and suicidal ideation. The aims of this work were to characterize MTK metabolism in vitro and in vivo and to identify its effects at the metabolome and proteome levels in order to explain its toxicity.
Main methods: An extensive study of montelukast metabolism was carried out using in vitro systems, an embryonic neuron-enriched cell model, and a mouse model. Metabolites were identified by high-resolution mass spectrometry, and a combined mass spectrometry-based metabolomics and proteomics approach was employed to assess the effect of MTK on mice and isolated chicken neurons.
Key findings: Eighteen new MTK metabolites were identified. MTK’s ability to react with glutathione was confirmed. The multi-omics approach employed confirmed that montelukast interferes with the glutathione detoxification system in the brain. Moreover, montelukast is also able to dysregulate various neurotransmitter and neurosteroid pathways, particularly those involved in regulation of the hypothalamic–pituitary–adrenal axis, also interfering with mitochondrial function in neuronal cells.
Significance: Results clearly indicate that montelukast therapeutic effects are accompanied by a strong modulation of specific processes in the central nervous system that may explain the observed neuropsychiatric reactions. Moreover, the results also suggest that adverse drug reactions are more likely to occur in children, due to the early maturation stage of their brains.”
Leukotriene receptor antagonists and risk of neuropsychiatric events in children, adolescents and young adults: a self-controlled case series
Ji Soo Park, Yoo Jung Cho, Je-Yeon Yun, Hye Jin Lee, Jinho Yu, Hyeon-Jong Yang, Dong In Suh
Eur Respir J 2022 Nov 3;60(5)
https://pubmed.ncbi.nlm.nih.gov/35595323/
“Background: Leukotriene receptor antagonists (LTRAs) are widely used for asthma and allergic rhinitis (AR), but concerns about the risk of neuropsychiatric events (NPEs) have been raised since the first Drug Safety Communication by the US Food and Drug Administration in 2008. This study evaluates the association between LTRA use and NPEs in children, adolescents and young adults with asthma or AR.
Methods: A self-controlled case series study was conducted using the Korean National Health Insurance Service claims database from two 3-year observation periods (observation period 1 (Obs1): 2005-2007; observation period 2 (Obs2): 2016-2018). Asthma or AR patients aged 3-30 years who were prescribed LTRAs and diagnosed with NPEs were included. The incidence rate ratios (IRRs) for the exposed period and risk periods (1-3, 4-7, 8-14, 15-30, 31-90 and >90 days from initiation of LTRA) compared with unexposed periods were calculated using conditional Poisson regression. Subgroup analysis according to age group, type of NPEs and indication of LTRA was performed.
Results: Among 17 001 included patients, the risk of NPEs increased in Obs2 (IRR 1.11, 95% CI 1.00-1.22), but did not increase in Obs1. Risk was increased during risk periods 4-7 days (IRR 2.36, 95% CI 1.99-2.76) and 8-14 days (IRR 1.78, 95% CI 1.46-2.15) after initiation of LTRA, particularly in adolescents (IRR 1.28, 95% CI 1.05-1.55) and young adults (IRR 1.14, 95% CI 1.02-1.28), while risk was decreased in children (3-11 years). Risk was not increased for any single type of NPE. AR patients were at increased risk (IRR 1.19, 95% CI 1.01-1.39), but not those with asthma.
Conclusions: Overall, risk of NPEs with LTRA use differed between risk periods and subgroups. Physicians should prescribe LTRAs according to indications and inform patients about possible NPEs.”
In Vivo and in Vitro Study of the Effect of the Anti-Asthmatic Drug Montelukast on DNA and Activity of Free Radical Scavenging Enzymes
Hassouneh K L, abudayeh H Z, Aldajah O S, Najdawi M M, Abualassal I Q, Altalhouni A A
Journal of Pharmaceutical Negative Results 2022;13(4): 1692-1698
https://www.pnrjournal.com/index.php/home/article/view/5497
“This work aimed to investigate the potentiality of Montelukast to alter DNA and the activity of free radical scavenging enzymes (FRSE) in mammalian blood serum and mice liver tissues. DNA was extracted from Balb/c mice pretreated with different concentrations of Montelukast (1.25, 2.5 and 5.0 mg/kg body weight (BW)) and human blood treated with (2.5, 5.0 and 10.0) µg/ml solution to examine the effects of Montelukast in vivo and in vitro, respectively. DNA damage was assessed using gel electrophoresis of S1-nuclease digested DNA to detect the presence of single strand breaks. To determine the effect of Montelukast on FRSE, the activity of glutathione s-transferase (GST), superoxide dismutase (SOD) and catalase (CAT) was measured in liver tissue and serum from Balb/c mice and in human blood treated with Montelukast as described above. Our investigation has shown that Montelukast may possess an indirect effect on DNA that is associated with decreased FRSE activity; the treatment was not associated with increased numbers of ss-breaks (single-strand breaks) in DNA in vivo or in vitro. However, GST, SOD, and CAT activity was significantly decreased compared to the untreated control (p < 0.05) in liver tissue in vivo from mice treated with 2.5 and 5.0 mg/kg BW Montelukast and in human tissue treated with 5 and 10.0 µg/ml Singular. Meanwhile, the enzymatic activity in vitro was significantly decreased (p < 0.05) in GST, SOD and CAT either mouse or human blood serum with increasing the concentration of Montelukast in liver tissue.”
Increased Risk of Tourette Syndrome with Leukotriene Modifier Use in Children with Allergic Diseases and Asthma: A Nationwide Population-Based Study
Min-Lan Tsai, Hsiu-Chen Lin, Chiung-Hui Yen, Jung-Tzu Ku, Shian-Ying Sung and Hsi Chang
Children October 2022, 9, 1607. https://doi.org/10.3390/children9111607
“(1) Background: Cysteinyl leukotriene receptor antagonists (LTRAs), including montelukast and zafirlukast, are FDA-approved for treating pediatric asthma and allergic diseases. Tourette syndrome (TS), a common neuropsychiatric disorder in children, is associated with allergic diseases and asthma. In this study, we investigated the risk of TS following an LTRA prescription for pediatric allergic diseases. (2) Methods: Children younger than 18 years of age who were newly diagnosed with asthma, allergic rhinitis, or atopic dermatitis between 1 January 2005 and 31 December 2018 and who were registered in the Taiwan National Health Insurance Research Database, which comprises the medical records of nearly 23 million Taiwanese population, were enrolled. LTRA users were matched with randomly selected LTRA non-users by sex, age, asthma-diagnosis year, and urbanization level. In total, 26,984 participants with allergic disease and TS were enrolled and included in the Cox proportional hazards model analysis. (3) Results: Children with allergic disease and asthma treated with LTRAs had a higher risk for TS than LTRA non-users (adjusted hazard ratio 1.376 [95% CI: 1.232–1.536], p < 0.001). LTRA users had a significantly higher risk for TS than LTRA non-users with allergic disease. The cumulative incidence of TS was significantly higher in LTRA users than in non-users with allergic diseases and asthma (log-rank test, p < 0.0001). (4) Conclusion: A prescription of LTRAs, mainly montelukast, increased the risk of TS among children with asthma, allergic rhinitis, or atopic dermatitis. The mechanism underlying the neuropsychiatric effect of LTRAs needs further investigation.”
Adverse Drug Reactions (ADRs) of Montelukast in Children
Al-Shamrani As, Alharbi S, Kobeisy S, Alkhater S, Alalkami H, Alahmadi T, Almutairi A, Alharbi A, Yousef A
Children, November 21, 2022
https://pubmed.ncbi.nlm.nih.gov/36421233/
“We retrospectively studied all adverse drug reactions to montelukast among 385 children 6 months or older in five tertiary centers over a two-year period. This study reported a high prevalence of adverse effects among 123 patients (31.9%), predominantly in those aged 4-9 years (52.8%), followed by adolescent children (24.4%) and toddlers (22.8%). Two adverse effects were reported in 9.8% of the children, while three or more were reported in 5.5%. Sleep disturbance was the most common adverse effect, affecting 15.1% of participants, followed by agitation (10.4%), pain (9.4%) and hyperactivity (6.8%). Although 81% of the families believed it was an effective and preventive medication, 5.3% stopped the drug due to concern about side effects, especially agitation (3%) and nightmares (0.6%). These data demonstrate that montelukast is effective, but the associated adverse neuropsychiatric drug reactions are more prevalent than those reported in the literature. Pediatricians should be aware of such adverse effects. Misconceptions about montelukast are still common, and parental counseling and urgent epidemiological studies are needed to quantify the risk for management plans.”
Leukotrienes vs. Montelukast—Activity, Metabolism, and Toxicity Hints for Repurposing
Cátia F. Marques, Maria Matilde Marques and Gonçalo C. Justino
Pharmaceuticals (Basel). September 2022 15(9): 1039.
“A growing number of MTK ADRs has been reported in the literature, focusing on neuropsychiatric aspects, especially anxiety and sleep disorders. In 2009, a total of 48 reports of psychiatric disorders in children were found in the Swedish ADR database SWEDIS. Nightmares, general anxiety, aggressiveness, sleep disorders, insomnia, irritability, hallucination, hyperactivity, and personality disorder were some of the most reported ADRs. Approximately 50% of these effects occurred in children under 3 years old and, in 80% of the reports, ADRs developed within 1 week from the first MTK administration. Later, a cohort of 14,670 individual case safety reports, of which 2630 corresponded to children and adolescents younger than 18 years old, were reviewed in 2015. The main conclusions highlighted children as the most likely to experience montelukast ADRs: sleep disorders were mostly reported in children younger than 2 years old; depression and anxiety signs in children between 2 and 11 years; and suicidal behaviour and depression/anxiety in adolescents between 12 and 17 years. Surprisingly, achieved suicides were more reported in children than adolescents or adults. Between 2012 and 2017, an observational study in a Spanish paediatric hospital concluded that 5.7% of children under 15 years old experienced ADRs, mainly insomnia, hyperactivity, and nightmares, which disappeared after MTK discontinuation.
Isolated cases of well-defined neuropsychiatric events in children and adults taking montelukast are also described in the literature. A 9-year-old boy experienced sleepwalking, sleep disturbance, bruxism, and anxiety during MTK treatment. After MTK withdrawal, the symptoms resolved without further intervention. Another case described a 13-year-old who experienced hallucinations that stopped 48 h after MTK withdrawal. A 16-year-old girl who was medicated with MTK reported parasomnias (sleeptalking and sleepwalking) during two attempts at MTK treatment. Symptoms stopped after MTK withdrawal for both attempts. A 29-year-old asthmatic woman suffered from visual and auditory hallucinations, which stopped two days after MTK withdrawal.”
Montelukast induces beneficial behavioral outcomes and reduces inflammation in male and female rats
Ira S. Rostevanov, Batya Betesh-Abay, Ahmad Nassar, Elina Rubin, Sarit Uzzan, Jacob Kaplanski, Linoy Biton, Abed N. Azab
Frontiers in Immunology, 6 September 2022
https://www.frontiersin.org/articles/10.3389/fimmu.2022.981440/full#T2
Though the direct intermediating immune-pathogenic mechanisms are still unofficially established, interactions between the immune system and the brain have attracted considerable attention in the field of neuropsychiatric diseases, and brain regions including the frontal cortex (FC), hippocampus (HC) and hypothalamus (HT) have been repetitively linked to such. Inflammatory mediators (such as prostaglandin [PG] E2, interleukin [IL]-6, and tumor necrosis factor [TNF]- α), which regulate brain function, proliferation, differentiation, and survival of brain cells, have also shown interconnection to psychiatric disorders…. MTK significantly increased IL-6 levels in the HT of control male rats…. in male rats: MTK significantly increased TNF-α levels in the FC in control animals…. MTK significantly decreased PGE2 levels in the FC and HT of CUMS-subjected males, but significantly increased its levels in the HC… generally, MTK did not adversely influence animal behavior in either control (non-stressed) or CUMS-subjected rats. An exception was the aggression-inducing effect of MTK in male rats. However, this negative effect of MTK is “counter-balanced” by its positive behavioral effects in males and females in other conditions….
a large body of data suggested that inflammation contributes to the pathophysiology of mental disorders. For example, several research papers reported increased IL-6 levels in patients with major depression, bipolar disorder and schizophrenia. Moreover, multiple studies showed that IL-6 levels were prominently increased in the blood of subjects after suicidal attempts and in post-mortem brains of people after suicidal death. Similarly, many studies found that TNF-α levels are higher in patients with major depression, bipolar disorder and schizophrenia than in matched-controlled subjects. PGs (PGE2 in particular) have been recurrently observed as connected to the pathophysiology of psychiatric disorders. These outcomes are highly relevant to the behavioral findings of the present work, because numerous studies have demonstrated that MTK decreases the levels of several pro-inflammatory mediators including IL-6, TNF-α and PGE2 under various experimental conditions. Thus, we hypothesized that the behavioral effects of MTK may be influenced by and related to its effects on brain inflammation.
In the present study we assessed brain inflammation by measuring inflammatory mediator levels in the FC, HT and HC. Table 2 summarizes the effects of MTK treatment and the exposure to CUMS on levels of IL-6, TNF-α and PGE2 in these brain regions. As seen, in control males, MTK increased IL-6 levels in the HT, and TNF-α levels it the FC, suggestive of a pro-inflammatory effect of the drug. In contrast, in control females, MTK treatment was associated with a robust anti-inflammatory effect; it significantly decreased IL-6 and TNF-α levels almost in all brain regions. On the other hand, MTK increased PGE2 levels in the FC and HC. These results are similar to those of previous studies which revealed that under certain conditions MTK may increase the production of PGE2. We speculate that the oppositional impact of MTK on IL-6 and TNF-α levels in male vs. female rats may contribute, at least in part, to its distinctive effect on aggressive-like behavior. MTK induced aggressive-like behavior in males, while it decreased this behavior in females. Numerous studies demonstrated that aggressive behavior in humans and rodents is affected by the function and structure of the FC, HT and HC….
the results support our hypothesis that treatment with MTK differentially affects levels of brain inflammatory mediators in male vs. female rats, which plausibly explains the dissimilar behavioral phenotypes of the sexes. Randomized, double-blind clinical trials in human subjects are necessary to directly determine the behavioral effectual capacity of MTK.
A database of pediatric drug effects to evaluate ontogenic mechanisms from child growth and development
Nicholas P Giangreco, Nicholas P Tatonetti
Clinical and Translational Resource and Technology Insights, Vol 3, Issue 3, August 12, 2022
https://pubmed.ncbi.nlm.nih.gov/35752163/
“Side effects are significant safety concerns in pediatric drug treatment but are rarely captured during clinical trials and are severely underreported post-market. Moreover, variations in metabolism and physiology as children grow and develop complicate detection of drug safety signals across child development. Researchers at Columbia University addressed this problem by inventing a novel algorithm for generating drug safety signals associated with and across child-development stages. They showed that their methodology reduced artificial drug and adverse event relationships, increased discovery of both known and rare side effects, and found evidence of different drug safety signals through development. They made their database of drug safety signals, called KidSIDES, freely available and browsable by the PDSportal web application … We investigate the relationship between development stage and known pediatric drug effects, such as montelukast-induced psychiatric disorders, where we found a significant signal (odds ratio 8.77 [2.51, 46.94]) within the second year of life … We discovered 32 “development-sensitive” medications, such as montelukast signal for psychiatric disorders, that exhibit dynamics in safety signals across childhood … For known culprits such as montelukast, our approach identified significant signals during mid-childhood (Figure 3), which is consistent with studies from the Swedish ADR database and the World Health Organization’s Vigibase.”
Montelukast-associated neuropsychiatric side-effects in tertiary paediatrics: a qualitative interventional study into awareness and prescribing practices
Flowers, S. and Minshall, E.
8th King’s John Price Paediatric Respiratory Conference, Royal College of Physicians, London, 16-17 June 2022
https://app.virtualpostersession.org/e/b76bd4bc6adbe8bd7111b3c85e53f86d
“Montelukast has been linked to severe neuropsychiatric side effects, prompting a recent government drug safety update. Prescribing clinicians and patients/carers may not always recognise that serious side effects can be linked to the drug.…An anonymous qualitative questionnaire, based on the recent drug safety recommendations, was sent to 51 clinicians in a tertiary paediatric children’s hospital…Depression and suicidal thinking/behaviour were initially under-appreciated as significant side-effects (45% awareness for both) but this improved (78% and 67% awareness respectively) following the educational material. The percentage of clinicians warning about depression, and suicidal thinking/behaviour also increased following the educational material (25% and 20% initially to 67% and 44% respectively) …Not all clinicians experienced in prescribing Montelukast are aware of potential neuropsychiatric side-effects. Knowledge of significant side-effects was sub-optimal, risking children’s well-being and mental health.”
Neuropsychiatric reactions with the use of montelukast
Ekhart C, van Hunsel F, Sellick V, de Vries T.
British Medical Journal 2022
“Montelukast, used in the treatment of asthma and allergic rhinitis, can cause serious mental health adverse effects such as nightmares, aggression, depression, and suicidal ideation. These adverse effects have been reported in patients of all ages, with and without pre-existing psychiatric disease, while taking montelukast or rarely after discontinuation. Inform patients and carers of these adverse reactions by discussing the patient information leaflet at the time of prescribing montelukast, and review within one month of initiation and regularly thereafter.”
Analysis of Neuropsychiatric Diagnoses After Montelukast Initiation
Paljarvi T, Forton J, Luciano S, Herttua K, Fazel S.
JAMA Network Open 2022
https://jamanetwork.com/journals/jamanetworkopen/article-abstract/2792596
“This propensity score–matched cohort study was conducted using electronic health records from 2015 to 2019 in the TriNetX Analytics Network patient repository of more than 51 million patients from 56 health care organizations, mainly in the US. Included patients were those aged 15 to 64 years at index prescription for montelukast or for control prescription who had a history of asthma or allergic rhinitis. After propensity score matching for various baseline confounders, including comorbidities and dispensed prescription medicines, we included 154 946 patients, of whom 77 473 individuals were exposed to montelukast. Patients were followed up for 12 months. Data were analyzed from June through November 2021…. In patients exposed to montelukast, the odds ratio [OR] for any incident neuropsychiatric outcome was 1.11 (95% CI, 1.04-1.19) in patients with asthma and 1.07 (95% CI, 1.01-1.14) in patients with allergic rhinitis compared with patients who were unexposed. The highest OR was for anxiety disorders (OR, 1.21; 95% CI, 1.05-1.20) among patients with asthma exposed to montelukast and insomnia (OR, 1.15; 95% CI, 1.05-1.27) among patients with allergic rhinitis exposed to montelukast…. These findings suggest that clinicians should consider monitoring potential adverse mental health symptoms during montelukast treatment, particularly in individuals with a history of mental health or sleep problems.”
FDA warning montelukast 03.2020 – Statement of the Austrian working group of pediatric pulmonology and allergology
Zschocke A, Horak F, Eber E, Frischer T, Simma B, Stetzl W, Riedler J, Szépfalusi Z, Zacharasiewicz A.. Wien Klin Wochenschr.
2022 Jan
https://pubmed.ncbi.nlm.nih.gov/34904177/
“The psychiatric side effects of montelukast have been known for the last 10 years; in the case of such symptoms benefits and risks should be considered. Due to potential life-threatening psychiatric adverse events, particularly suicide, a black box warning was issued. In this statement the Austrian working group of pediatric pulmonology and allergology advises that treatment with montelukast should be started only after critical evaluation. Treatment should be stopped on the occurrence of any neuropsychiatric side effects.”
World Allergy Organization: Ask the expert
Anti-leukotrine drugs, January 31, 2022
https://www.worldallergy.org/ask-the-expert/questions/anti-leukotriene-drugs
By Professors Claudia Renteria and Iris E. Hidalgo Nicho
For example, in a retrospective analysis of the World Health Organization (WHO) global database of individual case safety reports (ICSRs), 6722 ICSRs associated suicidal ideation with medications prescribed in the pediatric population; 674 (10%) of these reports corresponded to montelukast use. A similar study was conducted in Sweden, where ICSRs were recordered in subjects under 18 years of age from 2001 to 2010; 13 ICSRs were consistent with suicidal conditions; however, only 1 (8%) was associated with montelukast.
A review of suicide-related adverse events reported to the FDA was conducted for Montelukast, Zafirlukast, and Zileuton during the period 1998 to 2009. A total of 838 adverse events associated with leukotriene modifying agents (LTMAs) were notified; suicidal ideation was the most frequent event, followed by suicide attempt and death by suicide; montelukast was implicated in 833 (99.4%) events and Zileuton was involved in the remaining five cases.
Glockler-Lauf and colleagues examined the association between montelukast prescription and neuropsychiatric events in children. They found that nearly half (42.4%) of neuropsychiatric events occurred within 90 days of the most recent asthma maintenance prescription, and 22.2% of events occurred between 90 and 180 days from the prescription. In summary, children who used montelukast for asthma control had almost twice the odds of neuropsychiatric events compared to those who took other medications for asthma maintenance.
Neuropsychiatric adverse reactions associated with LTMAs are likely underreported in clinical trials because most trials focus on drug efficacy and are not powered or specifically designed for neuropsychiatric symptoms or events. Furthermore, the average follow-up time of people in some studies was less than 2 months, which may have underestimated the risk of neuropsychiatric events, given that the time to onset of these events can vary from hours to months after drug initiation.
Neuropsychiatric adverse drug reactions induced by montelukast impair the quality of life in children with asthma
Oznur Yilmaz Bayer, Ipek Turktas, Hacer Ilbilge Ertoy Karagol, Sebnem Soysal & Dilek Yapar
Journal of Asthma 2022
“Patients, ages 3–18 years, taking montelukast for the first time and their parents were included. Ages 3–7 years were defined as the preschool and ages 8–18 years as the school-age group. At the beginning of the study and at the end of the second week of treatment, the neuropsychiatric complaint assessment questionnaire and the KINDL QoL scale were administered to patients and their parents. The effect of ADRs on the decrease in QoL was evaluated by multivariable logistic regression. Neuropsychiatric ADRs were reported in 78 (62.4%) of 125 patients, who recovered when the drug was discontinued. Temperamental behavior, nightmares and sleep disorders occurred significantly more often in both groups compared with pretreatment (p < 0.001 for each). In both groups, except in the child-reported family relationships subscale in the school-age group, significant decreases were found in both child and parent proxy-reported QoL total/sub-scores compared with pretreatment (p˂0.001 for each). It was found in the evaluation that the overall QoL of those experiencing ADRs in both age groups was more affected. (Child-reported QoL ORpreschool age=2.66, p = 0.048; ORschool-age=5.95, p = 0.027; parent-proxy QoL ORpreschool age =3.52, p = 0.010, ORschool-age=6.43, p = 0.027). Montelukast-induced neuropsychiatric ADRs are more frequent than reported in the literature and negatively impact children’s QoL.”
Montelukast and Nightmares: Further Characterisation Using Data from VigiBase
Watson, S., Kaminsky, E., Taavola, H. et al.
Drug Safety 2022
https://link.springer.com/article/10.1007/s40264-022-01183-2#citeas
“Montelukast is a medicine indicated for use in asthma. Psychiatric disorders including nightmares have not been described in clinical trials but during recent years have been included in the product information as having been reported post-marketing, without further description of the events…. The study aim was to further characterise post-marketing adverse drug reactions for nightmares, suspected to be induced by montelukast, to facilitate safer use of the medicine by providing additional information to patients and healthcare professionals. We clinically reviewed reports of nightmares with montelukast present in VigiBase, World Health Organization’s global database of suspected adverse reactions to medicinal products, developed and maintained by the Uppsala Monitoring Centre, until 3 May, 2020. There were 1118 reports of nightmares with montelukast in VigiBase, which provided valuable descriptions of the nightmares as well as information about the impact on the daily lives, with many cases describing a severe impact of the nightmares. About half of the reports were classified as serious. Two thirds concerned children, with the largest age group represented being children aged 5–10 years. In most cases, the nightmares disappeared upon discontinuation of the drug but for some patients it took a long time until the nightmares ceased. The nature and potential severity of this adverse drug reaction, as described in these reports, present important knowledge for patients and healthcare providers that could help reduce drug-induced harm. This study highlights the value of post-marketing reports for further characterisation of known adverse drug reactions. The benefit–risk balance should be continuously monitored while patients are taking montelukast.”
Neuropsychiatric Event on Withdrawal of Montelukast
Els, I. and Webb, S.
Journal of Paediatric Child Health 2022
https://onlinelibrary.wiley.com/doi/full/10.1111/jpc.15937
“A 5-year-old-boy was treated for 12 months with montelukast, in combination with inhaled corticosteroids, after a life-threatening asthma attack. The parents were counselled prior to commencement regarding the potential for neuropsychiatric symptoms during treatment with montelukast. A decision was made by the parents to cease the montelukast a year later as the boy had had an event-free year. Five days after ceasing the montelukast, he developed severe agitation and anxiety, obsession with insects ‘crawling over him’, had nightmares and panic attacks and described auditory hallucinations. He has no prior psychiatric history and had been a well-adjusted social little boy. Over the following 8 weeks, his symptoms gradually improved, although he continued to have nightmares for some months. He required psychology intervention. No other stressors have been identified, nor is there any genetic susceptibility present. In March 2020, the United States Food and Drug Administration (FDA) strengthened existing warnings by requiring montelukast to have a Boxed Warning about serious mental health side effects.1 This warning includes the statement ‘Most reported cases of neuropsychiatric events occurred during montelukast treatment, but some occurred after discontinuation’ and ‘in some cases symptoms persisted after stopping montelukast or were reported after discontinuation of therapy’. This followed a high-level review of neuropsychiatric adverse events reported to the FDA Adverse Event Reporting System including data from January 1998 to January 2018. There were 15 cases of withdrawal neuropsychiatric events, six of which were new onset after withdrawal and nine were continued or recurring. Two additional cases, with limited information preventing robust assessment of causality, were identified between January 2018 and May 2019.2This case highlights the need for continued pharmacovigilance of montelukast, including awareness that neuropsychiatric events may occur as a withdrawal event.”
Adverse drug reactions of montelukast and pranlukast: Analysis of the Korea database
Shin EY, Jin JH, Kang MK, Yoo YS, Lee JH, Song WJ, Kwon HS, Cho YS, Moon HB, Kim TB.
Asian Pacific Journal of Allergy and Immunology 2022
https://apjai-journal.org/wp-content/uploads/2022/03/AP-030821-1202.pdf
“When prescribing montelukast and pranlukast, attention would need to digestive and sleep disorders, especially women aged 19 to 64. After prescribing montelukast, physicians would need to pay more attention to agitation (5/396378 vs 0/82475), bad or vivid dreams (6/396378 vs 0/82475), anxiety (11/396378 vs 0/82475), depression (14/396378 vs 1/82475), tremor (53/396378 vs 7/82475), irritability (5/396378 vs 1/82475), insomnia (159/396378 vs 25/82475), and headache (68/396378 vs 10/82475), compared to when prescribing pranlukast. Further prospective research needs to elucidate the relationship between neuropsychiatric events and montelukast.”
Neuropsychiatric Adverse Events of Montelukast: An Analysis of Real-World Datasets and drug−gene Interaction Network
Umetsu Ryogo, Tanaka Mizuki, Nakayama Yoko, Kato Yamato, Ueda Natsumi, Nishibata Yuri, Hasegawa Shiori, Matsumoto Kiyoka, Takeyama Noriaki, Iguchi Kazuhiro, Tanaka Hiroyuki, Hinoi Eiichi, Inagaki Naoki, Inden Masatoshi, Muto Yoshinori, Nakamura Mitsuhiro
Frontiers in Pharmacology, Vol 12, 2021
https://www.frontiersin.org/articles/10.3389/fphar.2021.764279
“Montelukast is a selective leukotriene receptor antagonist that is widely used to treat bronchial asthma and nasal allergy. To clarify the association between montelukast and neuropsychiatric adverse events (AEs), we evaluated case reports recorded between January 2004 and December 2018 in the Food and Drug Administration Adverse Event Reporting System (FAERS). Furthermore, we elucidated the potential toxicological mechanisms of montelukast-associated neuropsychiatric AEs through functional enrichment analysis of human genes interacting with montelukast. The reporting odds ratios of suicidal ideation and depression in the system organ class of psychiatric disorders were 21.5 (95% confidence interval (CI): 20.3–22.9) and 8.2 (95% CI: 7.8–8.7), respectively. We explored 1,144 human genes that directly or indirectly interact with montelukast. The molecular complex detection (MCODE) plug-in of Cytoscape detected 14 clusters. Functional analysis indicated that several genes were significantly enriched in the biological processes of “neuroactive ligand–receptor interaction.” “Mood disorders” and “major depressive disorder” were significant disease terms related to montelukast. Our retrospective analysis based on the FAERS demonstrated a significant association between montelukast and neuropsychiatric AEs. Functional enrichment analysis of montelukast-associated genes related to neuropsychiatric symptoms warrant further research on the underlying pharmacological mechanisms.”
Relationship between montelukast and behavioural problems in pre-school children with asthma
Eda Özata, Zülfikar Akelma, Sacit Günbey
Allergologia et immunopathologia, 31 December 2021
https://www.all-imm.com/index.php/aei/article/view/312/737
“This study was completed with 155 cases. The asthma group consisted of 95 children (45 in the ICS group and 50 in the montelukast group), and the healthy control group consisted of 60 cases…However, medication was discontinued in one asthmatic child due to developing hallucination 15 days after being started on montelukast. That patient was excluded from the study due to not meeting the inclusion criteria. We encountered no findings that montelukast results in a general increase in neuropsychiatric side effects. However, side effects may emerge incidentally in some patients. The parents of children started on montelukast must therefore be appropriately informed, and patients must be monitored in terms of potential side effects.”
Adverse drug reactions of montelukast: from theory to practice. Case report
Pilar Caudevilla Lafuente, Juan P García Íñiguez, Carlos Martín de Vicente
Archivas Argentinos de pediatria, 2021 Aug;119(4):e357-e359. (Article in Spanish, abstract in English)
https://pubmed.ncbi.nlm.nih.gov/34309318/
“Montelukast is widely used in recurrent wheezing and/or asthma treatment. Several adverse drug reactions (ADRs) have been described in children related to montelukast. Neuropsychiatric reactions are one of the most important. We designed an observational, retrospective, descriptive study on ADRs related to montelukast in the Pediatric Pulmonology Unit, Hospital Universitario Miguel Servet, Zaragoza, Spain. Between January 2012 and December 2017, in the Pediatric Pulmonology Unit, 348 patients were treated with Montelukast; of them, 20 presented RAM. The main symptoms described Reacciones adversas a montelukast: de la teoría a la práctica. Serie de casos Adverse drug reactions of montelukast: from theory to practice. Case report were insomnia (n = 7), hyperactivity (n = 4), nightmares (n = 3), abdominal pain (n = 2) and paraesthesia in extremities (n = 2). They appeared from the first days to months after the start of treatment and disappeared after stopping it. Two patients presented limb paresthesia, not described previously in children. The 5.7 % of our patients treated with montelukast had ADRs that required treatment discontinuation. Sleep disorders were the most frequent.”
Common asthma medicine has more side effects than thought
Finoulst, M and Vankrunkelsven, P
Tijdschrift voor Geneeskunde en Gezondheidszorg, June 2021
https://tvgg.be/nl/artikels/psychiatrische-bijwerkingen-van-montelukast-frequenter-dan-gedacht
(English abstract provided by authors)
“In a critical analysis of the 2009 Dutch Medicines Bulletin, author D. Bijl notes that clinical studies are not designed to investigate neuropsychiatric side effects. No specific questions are asked about effects on mood and behavior of treated children, which makes underreporting probable. In December 2020, the first prospective observational study, set up by the Department of Allergy and Asthma of the Gazi University in Turkey, on the occurrence of psychiatric side effects in 128 patients between 3 and 18 years (73.6% under eight years) treated with montelukast for asthma, was published. The results are staggering: 62.4% of all children treated, both younger and older, show psychiatric side effects shortly after the start of treatment. Behavioral changes, nightmares and sleep disturbances are the most common. The psychiatric side effects disappear within three days after stopping treatment with montelukast… Suicidal thoughts and suicide also occur. These unusual side effects have long remained under the radar and are still too little known. Prescribers should warn the parents of patients about the psychiatric effects of montelukast.”
Adverse drug reactions of leukotriene receptor antagonists in children with asthma: a systematic review
Dixon EG, Rugg-Gunn CE, Sellick V, et al
British Medical Journal Paediatrics Open2021
“‘Common’ adverse drug reactions (ADRs) included ‘agitation/hyperactivity/irritability/nervousness’, ‘aggression’ and ‘headache’. The case reports showed a similar pattern, describing 46 different ADRs experienced by a total of eight patients…LTRAs have a wide range of suspected ADRs in children and young people (CYP), predominantly gastrointestinal and neuropsychiatric disorders. Careful monitoring of CYP with asthma is required, both to assess and manage ADRs and to step treatment down when clinically stable.”
Neuropsychiatric side reactions of leukotriene receptor antagonist, antihistamine, and inhaled corticosteroid: A real-world analysis of the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS)
Sainan Bian, Lisha Li, Zixi Wang, Le Cui, Yingyang Xu, Kai Guan, Bin Zhao, Lianglu Wang, Jia Yin,
World Allergy Organization Journal 2021
https://www.sciencedirect.com/science/article/pii/S1939455121000880
“In this study, we aimed to analyze the characteristics of drug associated neuropsychiatric events, and compare the differences among different drug categories.
Disproportionality analysis and Bayesian analysis were used in data mining to identify suspected neuropsychiatric events associated with LTRA, H1-AH, and ICS based on the United States Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) from January 2004 to September 2020. Demographic information, time interval to onset, and death rates of LTRA, H1-AH, and ICS-associated neuropsychiatric events were also analyzed. A total of 9475 neuropsychiatric events were identified. The number of neuropsychiatric events related to LTRA, H1-AH, and ICS were 5201 (54.89%), 3226 (34.05%), and 1048 (11.06%), respectively. LTRA related neuropsychiatric events were more common in patients aged 4–6 years (18.66%)…. Montelukast was highly associated with neuropsychiatric events, with a high reporting odds ratio (ROR). Most neuropsychiatric symptoms occurred within the first 10 days after drug initiation (78.63% for LTRA, 91.39% for H1-AH, and 84.07% for ICS)…. LTRA associated neuropsychiatric events reported in FAERS were most frequent in 4 to 6-year-old children. Most reported cases occurred within the first 10 days after drug initiation…. More attention should be paid to specific patients treated with LTRA and H1-AH.”
The role of leukotriene modifying agent treatment in neuropsychiatric events of elderly asthma patients: a nested case control study
Kang SO, Min KH, Kim HJ, Kim TH, Kim W, Lee KE.
Asthma Research and Practice 2021
https://asthmarp.biomedcentral.com/articles/10.1186/s40733-021-00070-4
“This study suggests that elderly asthma patients prescribed LTMAs had a higher risk of NPEs than patients who were not treated with LTMAs. The risk was highest within 60 days after taking LTMAs. The risk of all three specific NPEs (sleep disorder, mood disorder, anxiety disorder) was increased by LTMA treatment in every recency and duration of drug treatment. Therefore, clinicians should be aware of the potential risks of NPEs, especially in the early stages of LTMA treatment.”
A Boxed Warning for Montelukast: The FDA Perspective
Clarridge K, Chin S, Eworuke E, Seymour S.
Journal of Allergy and Clinical Immunology: In Practice 2021
https://www.jaci-inpractice.org/article/S2213-2198(21)00307-X/fulltext
“The U.S. Food and Drug Administration (FDA) became aware of postmarketing reports of neuropsychiatric adverse events with Singulair (montelukast) use in 2007. Over the years, the FDA has conducted reviews of the clinical trial safety data, focused analyses of postmarketing reports, and reviews of the published literature. These activities have resulted in successive labeling updates and public communications. However, there has been continued concern among stakeholders about the risk of neuropsychiatric events and the lack of awareness among prescribers and patients/caregivers. On the basis of these concerns, the FDA embarked on another comprehensive review and also conducted a new observational study using claims data in the Sentinel Distributed Database. In September 2019, the FDA held a public Advisory Committee meeting to discuss its review and solicit recommendations from the panel regarding labeling and communication strategies. After careful consideration of the available data and feedback received during the FDA Advisory Committee meeting, the FDA required a boxed warning and a revision specifically for the allergic rhinitis indication to reserve use of montelukast to patients who have an inadequate response or intolerance to alternative therapies. Based on benefit-risk considerations, the asthma indication was not changed. To provide insight into the process and rationale for the required labeling changes, we provide an overview of the decisionmaking framework we used.”
Psychiatric side effects of Montelukast, Singulair
Lecture by Dr. Sara Dugan, Ask a Psychiatrist, Northeast Ohio Medical University
January 12, 2021
Research on the signal mining of adverse events of montelukast sodium based on FAERS
04 January 2021
https://sciforum.net/paper/view/8947
“Objective To conduct data-mining of montelukast-related adverse events after marketing to provide a reference for safe clinical medication. Methods We use reporting odds ratio (ROR) and proportional reporting ratio (PRR) methods to mine the adverse reaction signals of montelukast on the adverse reaction report data of 22 quarters from 2015Q1 to 2020Q2, extracted from the FAERS database. Results Totally 467 signals were detected with ROR and PRR, and the most relevant 50 preferred terms are conducted based on the signal strength and signal frequency, 55.32% of signals were not reported in the proved label. Adverse reaction signals of montelukast involve 27 systems and organs, in addition to psychiatric diseases, majority of adverse events included respiratory, thoracic, and mediastinal disorders and examination. Conclusion Clinical use of montelukast should pay attention to the patient’s neuropsychiatric symptoms, especially those not reported in the proved label, such as Separation anxiety disorder, Sleep terror and PANDAS. For patients with mental history, phenylketonuria and autoimmune diseases who use the montelukast,relevant workers should pay attention to monitoring to ensure safe and rational drug use.”
In vitro cytotoxicity of montelukast in HAPI and SH-SY5Y cells
Yu-Ting Tseng, Tynan M. Cox, Gary D. Grant, Devinder Arora, Susan Hall, Amelia J. McFarland, Jenny Ekberg, Santosh Rudrawar, Shailendra Anoopkumar-Dukie
Chemico-Biological Interactions May 2020
“This study aimed to investigate whether montelukast can induce neuroinflammation and neurotoxicity in microglial HAPI cells and neural SH-SY5Y cells. The present study also compared the effects of montelukast with a 5-lipoxygenase inhibitor (zileuton) and a cyclooxygenase-2 inhibitor (celecoxib) to better understand modulation of related pathways. HAPI or SH-SY5Y cells were treated with the indicated drugs (3.125 μM–100 μM) for 24 h to investigate drug-induced neuroinflammation and neurotoxicity. Montelukast induced cytotoxicity in HAPI cells (50–100 μM), accompanied with caspase-3/7 activation, prostaglandin E2 (PGE2) release, and reactive oxygen species (ROS) production. Whilst both montelukast and zileuton down-regulated CysLT release in HAPI cells, zileuton did not significantly affect cell viability or inflammatory and oxidative factors. Celecoxib decreased HAPI cell viability (6.25–100 μM), accompanied with increasing caspase-3/7 activation and ROS production, but in contrast to montelukast increased CysLT release and decreased PGE2 production. Similar to observations in HAPI cells, both montelukast and celecoxib (50–100 μM) but not zileuton produced toxicity in SH-SY5Y neuroblastoma cells. Similarly, CM from HAPI cells treated with either montelukast or zileuton produced toxicity in SH-SY5Y cells. The results of the current study show the capability of montelukast to directly induce toxicity and inflammation in HAPI cells, possibly through the involvement of PGE2 and ROS, and toxicity in undifferentiated SH-SY5Y neuroblastoma cells. The current study highlights the importance of consideration between benefit and risk of montelukast usage and provides references for future investigation on decreasing montelukast-related NEs.”
“This study provides the first in vitro evidence on Montelukast toxicity to microglial and neuronal cells. Provides evidence that this toxicity may be mediated by inflammatory response. Suggests that the inflammatory response and toxicity may contribute to neuropsychiatric events seen with montelukast use.”
“The in vitro study showed that this attenuation of neuroinflammation and protection from death occurred after neurons were exposed low concentrations of montelukast, that is 10 μM or less [40]. The results of the current study showed similar results at the respective concentrations however as the concentration of montelukast increased, an increase in PGE2 and ROS was observed, suggestive that the neuroinflammatory and neurotoxicity occurs only at higher concentrations. The concentrations used in literature were close to peak plasma concentration after 10 mg montelukast administration in humans.”
“However, the pharmacokinetic profile of montelukast showed that in children (6–11 months) received 4 mg montelukast, plasma montelukast concentration can reach 667 ng/mL (range 201–1058 ng/mL), which is about 89% higher than adults [54]. Moreover, it is reported that montelukast concentrations in animal brain were higher than in plasma 24 h post-intraperitoneal injection suggesting that accumulation of drug may occur in the brain tissue after repeated dosing [44]. The above-mentioned studies suggest the possibility of a slower clearance and a higher accumulation of montelukast in children as well as in brain and may be a possible factor in the higher incidence of NEs in this age group.”
Psychiatric adverse drug reactions in the paediatric population
Ekhart C, Vries TD, Hunsel FV
Archives of Disease in Childhood 2020
https://adc.bmj.com/content/105/8/749
“Reports submitted to the Netherlands Pharmacovigilance Centre Lareb from 2003 to 2016 were used to investigate drugs causing psychiatric ADRs in the Dutch paediatric population. These data were corrected for drug utilisation in order to correct the number of reports for the number of users of a drug… Lareb received 918 reports of psychiatric ADRs, which constitute 15% of the reports of ADRs in children. Drugs used for the treatment of ADHD (methylphenidate and atomoxetine) and drugs used for the treatment of asthma (montelukast and fluticasone) were the most frequently reported…. Real-world data on psychiatric ADRs in the Dutch paediatric population show a consistent pattern with what is known from drug labels and the literature. Reports of psychiatric ADRs should be taken seriously because of the impact on medication adherence and the well-being of the child and its family.”
Drug-induced tics: An observational postmarketing study
Touafchia, D, Montastruc, F, Lapeyre-Mestre, M, Rousseau, V, Chebane, L, Revet, A.
Human Psychopharmacology: Clinical and Experimental 2020
“While drug-induced tics have been described, in particular with neuroleptics, psychostimulants, or anti-epileptics, the strength and the direction of these associations are still debated. The aim of this study was to investigate the association between tics and drug exposure through a two-step analysis in two pharmacovigilance databases. We first performed a descriptive clinical analysis of cases registered in the French pharmacovigilance database (FPVD) from January 1985 to December 2018. We then performed a disproportionality analysis in VigiBase®, the WHO pharmacovigilance database, from January 1967 to June 2019, through the calculation of reporting odds ratio (ROR). The drugs most frequently associated with tics in the FPVD were methylphenidate, lamotrigine, montelukast, tramadol, mirtazapine, venlafaxine, aripiprazole, and risperidone. In VigiBase®, we found a significant ROR with methylphenidate (ROR 37.54, 95% confidence interval [CI] 34.81–40.48), montelukast (ROR 12.18, 95% CI 10.29–14.41), aripiprazole (ROR 7.40, 95% CI 6.35–8.62), risperidone (ROR 4.40, 95% CI 3.72–5.21), and venlafaxine (ROR 1.52, 95% CI 1.14–2.03). This postmarketing study confirmed a potential harmful association with methylphenidate (the highest association, as expected), aripiprazole, risperidone, lamotrigine, and venlafaxine and, interestingly, found a strong signal with montelukast, which, to our knowledge, had never been published before.”
Patient Safety: Aggression, Irritability, and Violence: Drug-induced Behaviors
Kathleen Golebiewski, Jeannette Y. Wick
University of Connecticut School of Pharmacy Continuing Education Program, February 15 2020
Table 1. Drugs Among Top 100 that May Cause Irritability, Agitation, or Aggression
Medication: Montelukast Risk: 2% Notes of interest: Montelukast’s drug monograph suggests that patients and prescribers should be alert for neuropsychiatric events….
Connecting the Dots: Immunomodulatory Drugs
Some other medications causing aggressive behavior have immunomodulatory effects—for example, montelukast. It is a selective leukotriene receptor antagonist, prescribed to manage asthma and allergy symptoms. Its most common adverse events are headaches, abdominal pain, and rash. In a retrospective analysis of all ADRs in people older than one year of age, epidemiologists looked at montelukast’s most serious and most often reported ADRs. The authors examined the reporting odds ratio (ROR). The ROR compares the rate of reporting a specific ADR in a drug in comparison to the rate of reporting the same ADR for all other drugs. The greater the ROR, the more times that specific ADR was reported for that drug. Aggression was one of the more frequently reported ADRs found using their global spontaneous reporting database, reported in a total of 1,101 of 17,723 total reports (approximately two of every 25 reports).43 Of the 1,101 reports of aggression, the ROR for patients under 19 years of age was 29.77%, or roughly one in four. This value is relatively high, pointing toward a strong relationship between the ADR and montelukast. Researchers theorize that neuropsychiatric symptoms—like agitation—were reported frequently because montelukast may have an effect on blood-brain permeability and modify neurotransmitter production. Recent studies support this theory. Researchers wonder if decreased blood brain barrier permeability modifies the expression of neurotransmitters in such a way that it causes agitation and aggression.
Impact of montelukast and fluticasone on quality of life in mild pediatric sleep apnea
International Journal of Pediatric Otorhinolaryngology,
Volume 125, October 2019, Pages 66-70
https://www.sciencedirect.com/science/article/pii/S0165587619303015
Research has shown improvement in apnea-hypopnea index in children with mild obstructive sleep apnea treated with anti-inflammatory medications. Data on quality of life outcomes in children receiving these medications is lacking. We aim to assess quality of life in children with mild obstructive sleep apnea treated with montelukast and fluticasone.
Children between 3 and 16 years old with mild sleep apnea (apnea-hypopnea index > 1 and ≤ 5) presenting to a pediatric otolaryngology clinic were recruited prospectively and treated with 4 months of montelukast and fluticasone. Subjects’ caregivers completed the OSA-18, a validated quality of life survey, at baseline and 4 months.
Thirty-one patients were included. Mean (SD) age was 6.8 (3.9) years. Most subjects (54.8%) were black and 48% were obese. Mean (SD) apnea-hypopnea index of the subjects was 2.8 (1.0). The mean (SD) baseline OSA-18 score was 60.2 (18.5), indicating a moderate impact of sleep disturbance on quality of life. Following treatment, there was significant improvement (p < 0.005) in mean OSA-18 score. Four children discontinued montelukast due to behavioral side effects.
Neuropsychiatric Events with Use of Montelukast in Pediatric Patients
FDA Briefing Document, Pediatric Advisory Committee Meeting, September 27, 2019
https://www.fda.gov/media/131035/download (Page 14)
1.4.4. Animal and Observational Literature Reviews Animal Studies The biologic mechanisms underlying the neuropsychiatric events associated with montelukast treatment are currently not well understood. However, evidence from animal studies suggests that montelukast could act directly on cells in the brain. Orally administered montelukast (10 mg/kg/day, 7 days) was detectable in brain tissue and cerebrospinal fluid (CSF) in rats 9, providing evidence for its ability to cross the blood-brain barrier. This evidence is consistent with experimental data obtained in rats during the nonclinical development of montelukast by Merck. Twenty-four (24)-hours after a single 10 mg/kg (oral) dose of [14C]montelukast, radioactivity in brain exceeded that in plasma. 10 Montelukast is protective in several animal models of acute CNS injury and stroke; the clinical significance of this data is not known. Eriksson et al.11 recently showed that montelukast inhibited cellular proliferation and maturation in the hippocampus of the intact juvenile mouse brain.
In this study, juvenile mice (postnatal day 19) were treated with montelukast (10 mg/kg/day, intraperitoneal injection) for 14 days. The total number of dividing cells (Ki-67+ ) was decreased by approximately 50% in the granule cell layer (GCL) of the dentate gyrus of the hippocampus. Total neurons and microglia (Iba1+ ) in the GCL were also decreased in montelukast treated animals relative to vehicle controls. Montelukast is a potent competitive antagonist (IC50 = 2.3 nM) at its target, the CysLT1 receptor. 12 However, expression of the CysLT1R in the normal human brain is very low/non-existent. Montelukast is also a competitive antagonist of (IC50 = ~60 nM) of GPR17, a G-protein coupled receptor which is expressed on neurons and glial cells in the human brain. 13,14 GPR17 is recognized as a regulator of oligodendrocyte development and remyelinating function. 15 Montelukast inhibition of GPR17 function on neurons and/or glial cells may contribute to the biologic processes underlying the observed neuropsychiatric events associated with montelukast treatment.
Neuropsychiatric disorder and Montelukast: a case report and VigiBase® analysis
Scholz I, Banholzer S, Haschke M
Archives of Disease in Childhood 2019
https://adc.bmj.com/content/104/6/e54.2
“An 11-year-old boy suffering from asthma presented to his pediatrician with an acute onset of nervousness, restlessness and irritability. The teacher noticed a decline in school performance with a reduced attention span. The patient had been treated with Salbutamol (Ventolin®) and Salmeterol/Fluticasone (Seretide®) for the last few years. A treatment with Montelukast chewable tablets was started four months ago. The ADR was reported to the Regional Pharmacovigilance Centre (RPVC) Bern. The termination of the therapy with Montelukast lead to an amelioration of the symptoms. According to the WHO-UMC Causality Categories,1 the causality of Montelukast and the described symptoms was classified as ‘probable’… Psychiatric disorders such as agitation, psychomotor hyperactivity (including irritability and restlessness), disorders of attention and memory impairment (and others) are listed as known ADRs of Montelukast.2 3 The WHO pharmacovigilance database VigiBase® lists a total of 20’897 ADR reports for Montelukast, of which 4’705 (22.5%) refer to nervous system disorders and 6’828 (32.7%) to psychiatric disorders. Within the group of nervous system disorders 256 (5.4%) reports of psychomotor hyperactivity, 232 (4.9%) reports of disturbance in attention and 91 (1.9%) reports of memory impairment were recorded.4 The most common symptoms in the group of psychiatric disorders are depression (1’311, 19.2%) and aggressive behavior (1’175, 17.2%). If psychiatric ADRs occur, the risks and benefits of Montelukast should be reassessed.”
Montelukast and Neuropsychiatric Events in Children with Asthma: A Nested Case–Control Study
S. Dresden Glockler-Lauf, Yaron Finkelstein, Jingqin Zhu, Laura Y. Feldman, Teresa To
The Journal of Pediatrics 2019
https://www.sciencedirect.com/science/article/abs/pii/S0022347619301982
“A matched, nested case–control design was used to identify cases and controls from a cohort of children aged 5-18 years with physician-diagnosed asthma from 2004 to 2015, in Ontario, Canada, prescribed an asthma maintenance medication. Cases were children with a hospitalization or emergency department visit for a neuropsychiatric event. Cases were matched to up to 4 controls on birth year, year of asthma diagnosis, and sex. The exposures were dispensed prescriptions for montelukast (yes/no) and number of dispensed montelukast prescriptions in the year before the index date. Conditional logistic regression was used to measure the unadjusted OR and aOR and 95% CIs for montelukast prescription and neuropsychiatric events. Covariates in the adjusted model included sociodemographic factors and measures of asthma severity. In total, 898 cases with a neuropsychiatric event and 3497 matched controls were included. Children who experienced a new-onset neuropsychiatric event had nearly 2 times the odds of having been prescribed montelukast, compared with controls (OR 1.91, 95% CI 1.15-3.18; P = .01). Most cases presented for anxiety (48.6%) and/or sleep disturbance (26.1%). Children with asthma who experienced a new-onset neuropsychiatric event had nearly twice the odds of having been prescribed montelukast in the year before their event. Clinicians should be aware of the association between montelukast and neuropsychiatric events in children with asthma, to inform prescribing practices and clinical follow-up.”
Neuropsychiatric side effects of montelukast
Thomas Kovesi
The Journal of Pediatrics, 7 June 2019
https://www.jpeds.com/article/S0022-3476(19)30585-2/fulltext
“Glockler-Lauf et al provide important information about the incidence of serious neuropsychiatric side effects (NPSEs) because of montelukast; however, there are important limitations. Our pediatric respirology clinic, which includes 6 clinicians, saw 1607 unique patients with asthma between March 26, 2018 and March 25, 2019, of whom 267 (16.6%) were treated with montelukast. We have seen NPSEs with montelukast, including anxiety, nightmares, agitation, increased unruly behavior, and restlessness. When NPSEs occur, the medication is either stopped in clinic, or, in many cases, by the family at home. We believe that although these side effects are not extremely rare, they are not common either. As these side effects, in our experience, are mild and resolve with discontinuation of the drug, no one in our group codes these conditions when billing. Similarly, other clinicians in the province probably also do not code for NPSEs during admissions or emergency department visits unless they were striking, prompted the encounter, and/or influenced length of stay. Thus, although this report provides an estimate of the incidence of severe NPSEs, it likely underestimates the frequency of milder events. In addition, requests for montelukast for older children to the provincial Ministry of Health were routinely denied (example of form letter available on request). Older children and adolescents will, therefore, be underrepresented in the drug benefits database even though NPSEs are likely more obvious in this age group. Although montelukast appears to approximately double the risk of severe NPSEs compared with other maintenance asthma therapies, mild NPSEs are likely even commoner.”
Psychotic symptoms associated with montelukast in a 21-month-old girl
Taşkırana, Gülseren; Adanırb, Aslı Sürer; Gürbüzb, Perihan Turhan.
Psychiatry and Clinical Psychopharmacology, suppl. Supplement 1; Istanbul Vol. 29 (2019): 145-146.
https://www.proquest.com/openview/bce85b3ced91380a2cb613417889670c/1?cbl=28708&pq-origsite=gscholar
“A 21-month-old girl was referred to our outpatient unit with agitation and fear symptoms and sleep disturbance. She began to fear from almost everything 1 month ago, be irritable and anxious, did not want to sleep; and her parents had severe difficulty in calming her down and make her sleep. For the last 2 days, she began to look at the walls with fear, as if she was seeing something scary, and cry. She also seemed to hostile to her baby doll. Family did not mention any physical or emotional trauma or any changes in the lifestyle. Her laboratory tests, neurological examination, cranial MRI and EEG were normal. It was learned that she had been diagnosed as asthma and bronchiolitis and been on montelukast 4 mg/day treatment for 2 months. Her clinical situation was evaluated as a psychotic process, with hallucinations, hostility and aggression. As montelukast had been known to cause hallucinations psychotic reactions, it was stopped and her symptoms ameliorated in a month without any psychiatric medication.”
Montelukast: neuropsychiatric adverse drug reactions in Tunisian asthmatic children
Sarra Ammari, Anissa Berraies, Besma Hamdi, Emna Ben Jemia, Jamel Ammar, Agnès Hamzaoui
European Respiratory Journal 2018
Introduction: Montelukast is a widely prescribed treatment in childhood asthma. But, in 2017, two large studies confirmed that it was associated with neuropsychiatric adverse drug reactions (ADR), about one-third of which concerned children. The aim was to analyze the frequency of psychiatric ADR that happened in children taking Montelukast for their asthma and to describe the characteristics of patients with these ADR.
Methods: We collected by means of phone call all neuropsychiatric ADR in 56 children followed for asthma and receiving Montelukast.
Results: The mean age was 7.03years (2-17 years). Asthma was moderate to severe in 36 % of cases. All children received Montelukast as an add-on therapy with inhaled steroids. As in literature, neuropsychiatric ADR were reported in 32% of cases. They happened 2.75 weeks (1-24 weeks) after treatment initiation, and in 21% of the cases, before 2 weeks. The majority of ADR were benign: irritability (25%), agitation (18%), aggressiveness (20%), anxiety (12%), nightmares (12%), insomnia (4%) and drop in school performance (3%). No suicidal attitude or ideas were reported. Treatment was stopped by parents in 2 cases (3%) because of extreme aggressiveness, with a good outcome. Asthma was non-controlled in these two cases. For the other children, treatment was not interrupted because side effects were transient. Age< 4 years and asthma severity were not associated with the occurrence with the psychiatric ADR.
Conclusion: neuropsychiatric ADR in children taking Montelukast were frequent but not serious. The question of whether these ADR are exclusively related to Montelukast or may be influenced by the fact of living with a chronic disease is left open
The Anti-Asthmatic Drug, Montelukast, Modifies the Neurogenic Potential in the Young Healthy and Irradiated Brain
Yohanna Eriksson, Martina Boström, Åsa Sandelius, Kaj Blennow, Henrik Zetterberg, Georg Kuhn and Marie Kalm
Cell Death and Disease 2018
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6039496/
“Radiotherapy is an effective treatment for brain tumors but unfortunately causes long-lasting side effects in the brain. In this study, parameters that are known to be affected by [Cranial Irradiation] CIR were investigated to evaluate the effect of montelukast on radiation-induced injury in the developing brain. The major findings were the following: (1) Montelukast treatment resulted in reduced cell death during the acute phase after CIR. (2) The number of neurons was altered by montelukast treatment with a positive effect after CIR, but with a negative effect during normal conditions… An in vitro study showed an increased proliferation of neuronal progenitors after montelukast administration, but using higher doses it decreased proliferation29. This support our finding that montelukast could have a negative effect on neuronal proliferation in the intact juvenile brain… Whether montelukast is beneficial or not seems to depend on the absence or presence of injury in the young brain. Positive results from montelukast treatment have been seen in the aging brains of rats after a dose of 10 mg/kg body weight7. The dose to treat asthma in children (2–5 years old) is however 4 mg/day regardless of body weight. In this study, we have examined the effects of montelukast in the young brain after CIR at the same dose as in the aging study, 10 mg/kg, but during a shorter time course compared to the clinical situation. Our aim was to investigate if this dose of montelukast could have similar protective effects after CIR, as the damage resembles the aging brain with elevated inflammation and less proliferation. Nevertheless, our findings could also contribute to explaining some alterations that montelukast induce in the young healthy brain as we have seen negative effects both acutely and after 2 weeks of daily administration of montelukast. However, more studies are needed to explore the therapeutic window for montelukast in pediatric patients… In summary, montelukast has negative effects on the maturation of the GCL during normal conditions, whereas during a pathological condition, such as following CIR, the effects can be protective. These findings, with the affected proliferation during normal conditions, in combination with the new profile for psychiatric adverse drug reactions, suggests that prescribing montelukast to young children should be a well thought through decision. However, more studies are needed to investigate if the negative effects are occurring also at lower dose spans and if the effect is chronic if ending the treatment with montelukast.”
Similar adverse events from two disparate agents implicate lipid inflammatory mediators for a role in anxiety states
Gordon C McCarter, Lauren B Blanchard
Oxford Medical Case Reports, Volume 2017, Issue 11, November 2017, omx060, https://doi.org/10.1093/omcr/omx060
https://academic.oup.com/omcr/article/2017/11/omx060/4670558
The patient is a 56-year-old male in very good health. He exercises regularly, primarily walking, running, and bicycling, and has an excellent cardiovascular profile with a body mass index of 23. He is educated at the doctoral level and is medically literate….
Two to three weeks after initiating montelukast at 10 mg/day he noticed gradually worsening generalized anxiety and began waking suddenly after several hours of sleep. During his awakenings, he often experienced strong sympathetic activation, with increased heart rate, flushing and a loosening sensation in his viscera. These panic symptoms were associated with ruminative thoughts in which routine life concerns led to the imagination of catastrophic outcomes for himself, his family and society at large. He described one night in which he experienced nasal congestion and had persistent, intrusive fears of suffocation should his mouth somehow be forced shut. He is experienced in somatic quieting and cognitive self-soothing but found these difficult to execute in these night-time panic episodes…. The anxiety symptoms occurred virtually every night by the fourth week of taking montelukast.
The patient reported that in the daytime he was also frequently overcome by morbid fears of such things as aging in loneliness, his children failing at life or worldwide economic collapse. At other times he would suddenly feel extreme anxiety about expectations of him at his workplace, in which he experienced a wave of sympathetic arousal, mainly felt as flushing and an increased heart rate. Because of the night-time awakenings he was chronically sleep-deprived during this period, to which, based on experience, he attributed the fact that he felt restless and ‘jittery,’ and had a pounding heart throughout much of the day. The patient recognized that the current symptoms were very similar to those associated with fish oil supplements, but he considered them significantly more intense than the previous episode.
The patient did not associate his anxiety symptoms with the new medication until it was time to refill his prescription. He researched the adverse events associated with montelukast and immediately decided to discontinue the drug after ~1 month of daily use. The panic symptoms during night-time awakenings abated within a few days, while the awakenings themselves and the general anxiety took a few weeks to largely resolve. Several months after the discontinuation of montelukast the patient reported that he felt a ‘normal’ mild degree of daytime anxiety and experienced mild maintenance insomnia less than once a week with no panic symptoms.
Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice
Brigitte Benard, Valerie Bastien, Benjamin Vinet, Roger Yang, Maja Krajinovic, Francine M. Ducharme
European Respiratory Journal August 2017
“Although montelukast is generally well tolerated, postmarketing studies have reported serious neuropsychiatric adverse drug reactions (ADRs) leading to a United States Food and Drug Administration warning. The objective of this study was to determine the incidence of neuropsychiatric ADRs leading to discontinuation of montelukast in asthmatic children. We conducted a retrospective cohort study in children aged 1–17 years initiated on montelukast. In a nested cohort study, children initiated on montelukast as monotherapy or adjunct therapy to inhaled corticosteroids (ICS) were matched to those initiated on ICS monotherapy. A non-leading parental interview served to ascertain the occurrence of any ADRs with any asthma medication, and circumstances related to, and evolution of, the event. Out of the 106 participants who initiated montelukast, most were male (58%), Caucasian (62%) with a median (interquartile range) age of 5 (3–8) years. The incidence (95% CI) of drug cessation due to neuropsychiatric ADRs was 16 (10–26)%, mostly occurring within 2 weeks. Most frequent ADRs were irritability, aggressiveness and sleep disturbances. The relative risk of neuropsychiatric ADRs associated with montelukast versus ICS was 12 (2–90).
In the real-life setting, asthmatic children initiated on montelukast experienced a notable risk of neuropsychiatric ADRs leading to drug cessation, that is significantly higher than that associated with ICS.”
Neuropsychiatric adverse effects of montelukast in children
Pierre Ernst, Genevieve Ernst
European Respiratory Journal August 2017
https://erj.ersjournals.com/content/50/2/1701020
Neuropsychiatric ADRs of montelukast are common and difficult to recognise in children
Asthma is the most common chronic disease of childhood…. Montelukast, as a daily controller medication, provides efficacy similar, although less potent, than ICS with the possibility of improved adherence such that the overall clinical benefit may be equal [2]. Montelukast is therefore frequently used as the initial controller therapy as well as in addition to low-dose ICS…. In this issue of the European Respiratory Journal, Benard et al. [4] examine the frequency and relative risk of neuropsychiatric adverse events in children prescribed montelukast, as compared to ICS, in the real-world setting of a paediatric asthma clinic.…The median age was 5 years with 75% being ≤8 years old. The primary outcome, the incidence of neuropsychiatric ADRs serious enough to lead to cessation of therapy, was reported by 16%, or 12% if considering only ADRs definitely or probably related to montelukast.
All ADRs were judged to be mild or very mild and only required cessation of therapy. The ADRs reported were irritability, aggressiveness and sleep disturbances. There was no report of suicidal ideation. These adverse events occurred soon after initiation of therapy (median 7 days) and resolved quickly after stopping the medication (median 2 days)…. When comparing 84 children initiating montelukast to 84 children initiating ICS at a similar time, those initiating montelukast were estimated to be 12 times as likely to experience a neuropsychiatric ADR, although the confidence interval was wide (relative risk 12.0, 95% CI 1.60–90.2).… Notably, such adverse events were not reported in the initial clinical trials which led to licensing of this medication in children nor in the meta-analysis of these trials [7–9].That neuropsychiatric adverse events were not reported in the paediatric clinical trials of montelukast is surprising and disturbing…. There are several implications of the study by Benard et al. [4]. Firstly, clinical trials do not, by themselves, adequately assess the occurrence of adverse effects of medications. It remains the responsibility of the treating physician to have easy access to up-to-date online tools to check for reported adverse events and to engage patients actively to report possible adverse drug effects. At a meeting of the Pediatric Advisory Committee of the FDA held in September 2014, it was suggested that many healthcare professionals were unaware of the neuropsychiatric adverse events related to use of montelukast and that steps to increase awareness were required.
Given that neuropsychiatric symptoms in children may be attributed to a wide variety of conditions and possible triggers, it is imperative that knowledge of this common ADR of montelukast be disseminated not only to paediatric respirologists but also to paediatricians and primary care providers. However, physician education alone is unlikely to result in sustained change in prescribing practices. Monitoring for neuropsychiatric ADRs should be integrated into existing asthma guidelines and individual clinics should explore ways to remind physicians to ask families about this important ADR. Moreover, patients should not only be asked about common ADRs at follow-up visits but there also needs to be a system in place for families to report new concerns to their physician as they arise. The transition to electronic medical records in outpatient clinics will provide great opportunities for ongoing ADR surveillance and automated physician alerts. Most importantly, empowering families to report common ADRs on an ongoing basis would allow their physician to adjust their treatment plan sooner and may improve compliance, in addition to overall patient satisfaction.
As for the use of montelukast in children, adverse neuropsychiatric effects are sufficiently common and potentially difficult to recognise in young children to require that parents be informed of these at the initiation of treatment. Notably, the current study included only a small number of adolescents, such that the risk of suicidal behaviour in adolescents reported with montelukast cannot be quantified by the study by Benard et al. [4]. However, it may be prudent to discuss the possibility of mood changes as well as warning signs for suicidality with both adolescents and their parents when initiating treatment.”
Adverse drug reactions of montelukast in children and adults
Haarman, M. G., van Hunsel, F., de Vries, T. W.
Pharmacology Research and Perspectives 2017
“Montelukast, a selective leukotriene receptor antagonist, is recommended in guidelines for the treatment of asthma in both children and adults. However, its effectiveness is debated, and recent studies have reported several adverse events such as neuropsychiatric disorders and allergic granulomatous angiitis. This study aims to obtain more insight into the safety profile of montelukast and to provide prescribing physicians with an overview of relevant adverse drug reactions in both children and adults. We retrospectively studied all adverse drug reactions on montelukast in children and adults reported to the Netherlands Pharmacovigilance Center Lareb and the WHO Global database, VigiBase® until 2016. Depression was reported most frequently in the whole population to the global database VigiBase® (reporting odds ratio (ROR) 6.93; 95% CI: 6.5–7.4). In the VigiBase®, aggression was reported the most in children (ROR, 29.77; 95% CI: 27.5–32.2). Headaches were reported the most frequently to the Dutch database (ROR, 2.26; 95% CI: 1.61–3.19). Furthermore, nightmares are often reported for both children and adults to the Dutch and the global database…. These data demonstrate that montelukast is associated with neuropsychiatric adverse drug reactions such as depression and aggression. Especially in children nightmares are reported frequently.”
Case report: Cross roads between Pulmonology and Psychiatry
M. Ajit, K. Lokesh Kumar, Sandeep Krishnamurthy. K
Telangana Journal of Psychiatry, July-December 2017:3(2):121-124
https://www.ipinnovative.com/journal-article-file/5384
A 12yr old female child, telugu speaking, of rural dwelling, presented to psychiatry outpatient department along with her parents. The patient’s chief complaints were hearing of voices, audible thoughts and anxiety. On enquiring with the parents they reported that child was complaining that someone is calling her name frequently but when she checks nobody is around and
that she could hear her own thoughts along with frequent reports of anxiety since past 10 days. Her sleep and appetite were however normal. She was a known case of asthma and was recently prescribed Montelukast once daily. On examination the patient was alert and oriented with normal psychomotor activity. Mood was anxious, thought echo and second person auditory hallucinations were present, with intact cognitive functions. According to ICD 10 she was diagnosed as a case of acute schizophrenia like psychotic disorder and was started on Risperidone 2mg and Trihexyphenidyl hydrochloride 2mg. On review after two weeks her symptoms persisted. As Montelukast can cause adverse effects like hallucinations, mood changes and anxiousness, the diagnosis was revised to substance induced psychosis. Montelukast, Risperidone 2mg and Trihexyphenidyl hydrochloride 2mg were stopped and the patient was asked to come back for review after 10 days. On her next review her symptoms had remitted completely…. the complete remission of symptoms on stopping montelukast, even in the absence of
antipsychotic drug treatment weighs more in favor of drug induced psychotic symptoms. The psychiatric adverse effect of other prescribed medications is often overlooked while diagnosing psychiatric disorders, leading to an erroneous diagnosis of a primary psychiatric disorders. This case report emphasizes the need for a thorough evaluation of all comorbid conditions and the medications prescribed for the same, along with their adverse effect profile while evaluating a psychiatric disorder.
The effect of montelukast on depression behaviour in rat forced swimming test
Ersöz Gonca
Journal of Mood Disorders (JMOOD 2017;7(2):104-9 March 2017
https://www.ejmanager.com/mnstemps/8/8-1474726863.pdf?t=1671162650
“Conclusion: Montelukast treatment causes depression behavior in both healthy and asthmatic rats”
Psychiatric Disorders and Montelukast in Children: A Disproportionality Analysis of the VigiBase®
Aldea Perona, A., García-Sáiz, M. & Sanz Álvarez, E.
Drug Safety 2016
https://link.springer.com/article/10.1007/s40264-015-0360-2
(This table from the article shows that 8 children under 12 and 38 children between 12 and 17 were reported to have completed suicide)
“In 2008, the US FDA issued an alert about an increased risk of psychiatric events associated with montelukast. Recent national pharmacovigilance analyses in Sweden, France and Spain detected a potential increase in reporting risk of the association…. We conducted a retrospective analysis of Individual Case Safety Reports (ICSRs) recorded up to 1 January 2015 in the World Health Organization (WHO) database (VigiBase®), in which montelukast was associated with ‘psychiatric disorders’. We used the Bayesian Confidence Propagation Neural Network (BCPNN) approach for signal generation. A total of 14,670 ICSRs for montelukast were recorded, of which 2630 corresponded to psychiatric disorders in people aged <18 years. The main symptoms reported for infants (aged <2 years) were sleep disorders, for children (aged 2–11 years) the main symptoms were depression/anxiety, and for adolescents (aged 12–17 years) they were suicidal behaviour and depression/anxiety. Suicidal behaviour was over-represented in all age groups with information component (IC) values that reached 5.01 in children and 3.85 in adolescents. Unexpectedly, completed suicides were reported more frequently for children (IC: 3.15; IC025: 1.98) than for adolescents (IC: 3.11; IC025: 2.61) or the total population (IC 1.95; IC025: 1.73). Neuropsychiatric disorders as side effects of montelukast were more frequently reported for children than for adults. Infants and children seem to be more prone to sleep disturbances, whereas adolescents present symptoms of depression/anxiety and psychotic reactions more often. Suicidal behaviour and completed suicide appear to be more frequently reported than previously thought in practice. Risk management plans and epidemiological studies are needed to quantify the risk. Practitioners should be aware of the risk of neuropsychiatric events associated with montelukast use, and should advise the patient and report new cases.”
Montelukast-induced metamorphopsia in a pediatric patient: A case report and a pharmocovigilance database analysis
Carla Carnovale, Marta Gentili, Stefania Antoniazzi, Sonia Radice, Emilio Clementi
Annals of Allergy, Asthma and Immunology, February 10, 2016
https://pubmed.ncbi.nlm.nih.gov/26874930/
“A 12-years-old female suffering from asthma was treated with montelukast at the therapeutic dose (5 mg/day). Approximately an hour after the first oral administration of the drug, the patient experienced visual disturbance in which the perfectly straight lines appeared wavy, parts of the line appeared blank with flat surface bending. This visual disturbance lasted approximately 15 minutes. The patient was hospitalised. The neurological examination and the electroencephalogram revealed no abnormalities. The positive Amsler test confirmed the diagnosis of metamorphopsia. The patient received no other concomitant drug or herbal treatment and had no personal or family history of ocular diseases. Considering the temporality between the drug intake and the appearance of the reaction, the treatment was discontinued and a diagnosis of iatrogenic metamorphopsia was performed by the clinician. The ADR resolved after drug withdrawal in few days. The patient no longer experienced ocular disturbances and remained metamorphopsia-free during a 3-month follow-up. The positive dechallenge, the temporal association between drug’s use and the onset of the reaction suggested a possible causal relationship between the metamorphopsia and montelukast administration, confirmed also by the Naranjo Algorithm. Analysis of Pharmacovigilance databases Montelukast-associated metamorphopsia has not previously been reported in the literature [8], although the main International pharmacovigilance databases contain several reports of ocular event referred to the leukotriene-receptor antagonist. We retrieved 719 reports of ocular ADRs in which montelukast was the suspected drug involved, inserted into the following pharmacovigilance databases: the Danish Health and Medicines Authority, the Health Canada Vigilance Adverse Reaction Online Database, the Netherlands Pharmacovigilance Centre Lareb Databank, the UK Medicines and Healthcare products Regulatory Agency, the FDA Adverse Event Reporting System (AERS), the Australian Adverse Event Reporting System (DAEN) (Table 1). Of 719 ocular ADR reports 4 of those in the AERS described metamorphopsia, representing the 0.5% of the total ocular events retrieved in our analysis. Of these, one refers of a 13-year-old male with multiple allergies who was placed on therapy with montelukast (strength, formulation and indication not reported). Approximately 5 minutes after montelukast administration, the patient experienced visual disturbance with metamorphopsia. The limit of this analysis is that the pharmacovigilance databases only receives reports of the most critical and severe cases; these numbers we retrieved may underestimate the complication rate of the drug…Our current case report, along with previous data form literature and the International pharmacovigilance databases, identify in metamorphopsia a possible ADR by montelukast and provide a rationale for its occurrence. These results provide also means to help the physician to identify possible correlations between ocular side effects and montelukast for a prompt identification. An increased attention in pharmacovigilance to this ADR appears relevant because of the wide paediatric use of montelukast.”
Montelukast’s Underrecognized Adverse Drug Events
Editorial collaboration between Medscape and US FDA, March 02, 2015
https://www.medscape.com/viewarticle/840302?src=smo_tw_aimm%3Fsrc%3Dsttwit#vp_1
“Medscape: Why do you think this now 5-year-old safety issue is not well known by prescribers?
Dr Torjusen: That is a good question. It is possible that awareness of the association between montelukast and neuropsychiatric events has faded. HCPs are inundated with new products and new safety information, and keeping up with all of this can be a challenge. In addition, the types of events experienced are highly variable. Detecting these events in children and young adults can be particularly challenging. Young children may be less able to articulate these experiences, and parents may unknowingly attribute symptoms to be part of developmental changes. Therefore, reminders of important safety concerns may be necessary at times for both patients and providers.
Medscape: What are the key take-home messages for clinicians who are prescribing this class of drugs?
Dr Torjusen: The key take-home message is for HCPs to be aware of the risk for neuropsychiatric events with the use of montelukast. Providers should inform their patients and families that these types of side effects are a possibility with these medications, and they should follow up with their patients after initiation of therapy.
Patients should also notify their HCPs if side effects occur, and HCPs should consider discontinuing therapy if patients develop neuropsychiatric symptoms.”
Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug
Marschallinger, J., Schäffner, I., Klein, B., Gelfert, R., Rivera, F.J., Illes, S., Grassner, L., Janssen, M., Rotheneichner, P., Schmuckermair, C., Coras, R., Boccazzi, M., Chishty, M., Lagler, F.B., Renić, M., Bauer, H.C., Singewald, N., Blümcke, I., Bogdahn, U., Couillard-Després, S., Lie, D.C., Abbracchio, M.P., & Aigner, L.
Nature Communications, 6. 27 October 2015
https://www.nature.com/articles/ncomms9466.pdf
“For drug safety reasons, and for the interest on potential effects of montelukast treatment on noncognitive behaviours, we analysed changes in body weight (Fig. 1h), exploration and locomotion (Fig. 1i), anxiety-like behaviour (Fig. 1j) and depression-like symptoms (Fig. 1k). Montelukast treatment did not have any effect on these parameters, with the exception of a slight but significant increase in the time spent in the closed arms in the elevated plus maze in young animals, possibly pointing towards a slight anxiogenic effect of montelukast in the young group…. we treated young (4 months) and old (20 months) rats for 1 week orally with montelukast (10 mg kg 1 body weight) and analysed its presence in serum, brain and cerebrospinal fluid (CSF) by high-performance liquid chromatography 1 h after the last montelukast administration. As expected, montelukast was present in the serum of both young and old rats. Remarkably, montelukast was, although to a lower level, also detected in the brain and in the CSF of the treated animals (Supplementary Fig. 1a). Most importantly, in a human subject taking 10 mg per day montelukast, that is, the approved dose to treat asthma, we detected montelukast in the serum and in the CSF in a similar concentration as in the rats (Supplementary Fig. 1a), suggesting that the standard 10 mg per day dose in humans is sufficient to reach a therapeutic dose in the CSF. In addition, a re-analysis of the original CNS pharmacology data of montelukast indicates a significant BBB penetrance of the drug (Supplementary Fig. 1b). These data clearly demonstrate that orally administered montelukast does cross the BBB in a therapeutic dose, and that age-dependent differential BBB integrity does not affect the capacity of montelukast to enter the brain…. Remarkably, montelukast serum levels of the rats used in this study (young: 288±57.18 ng ml 1; old 365±56.45 ng ml 1; Supplementary Fig. 1) were almost identical to the maximum plasma concentrations in humans after oral administration of the clinical dose of 10 mg montelukast daily (385±85 ng ml 1) 27, illustrating that the animals were treated with montelukast in a dose that pharmacologically resembles the one that is approved for its use in humans… Although montelukast was so far always considered as a drug with only limited CNS penetration, careful re-analysis of the original pharmacokinetic report on montelukast reveals that one hour after i.v. drug administration, a substantial amount of radioactive equivalents of [C14] montelukast (B1/10 of the plasma levels) had reached the brain (Supplementary Fig. 1b). Most remarkably, while in plasma (and most other organs, for example, lung and muscle) montelukast levels strongly decreased within 24 h, the amount of montelukast in the brain increased. As a consequence, 24 h after drug injection, montelukast levels in the brain were even higher than in plasma (Supplementary Fig. 1b), suggesting the existence of an active transport mechanism for montelukast through the BBB.”
Mood and Behavioral Changes Associated with Montelukast Usage in Pediatric Cases
Banu Gulcan Oksuz, MD, Mahir Igde, MD, Onur Ozturk, MD
Indian Journal of Medical Research and Pharmaceutical Sciences (Vol.2, No. 2) 2015-02-28
https://www.researchgate.net/publication/271330558
“Conclusions: Patients who are mentally stable before montelukast treatments may have some disturbances in mood and behaviors during treatments so attention
should be paid for such reactions. Clinicians should suspect if the patient has unexpected reactions after montelukast. And further studies are needed to evaluate
these datas.”
Highlights of montelukast in Children. updated analysis of the related suicide behaviours in the who database. January 2015.
Aldea-Perona, A. ,Garcis-Saiz, M., Sanz-Alvarez, E.
Clinical Therapeutics, Volume 37, Issue 8, Supplement E42, August 2015
“The BCPNN method was used to analyse the ICSR of the association montelukast-suicide behaviours, up to January 2015, in order to evaluate the safety concerns that have been raised regarding the increased risk of completed, ideation and attempt suicide with its use. The most frequent fatal cases reported with Psychiatric disorders and montelukast were (Nfatal/Ncomb): 183/183 completed suicide, 26/1083 depression, 10/832 insomnia, 10/815 anxiety. The ICSR of suicidal ideation, aggression, suicide attempt or depression have positive dechallenge in 52 %, 44%, 22% and 48%, of the cases respectively. Positive rechallenge were reported in 3 cases of suicide ideation.
In the global WHO Database there were 6,722 ICSR for the “Suicidal and self-injurious behaviours” HLGT for all drugs in pediatric population. Out of them 10% (674) corresponds to Montelukast. These 10% reports included several PT: Suicidal ideation (447; 66%), Suicide attempt (95; 14%), Intentional self-injury (60; 9%), Completed suicide (56; 7%), Self-injurious ideation (44; 7%), Self-injurious behaviour (44; 7%) and, Suicidal behaviour (27; 4%). Within the “Suicidal and self-injurious behaviours” HGLT, Completed suicide PT (82%) and Suicide attempt PT (78%) were more frequently reported for adolescents and suicidal ideation (66%) for children (2-11y). The IC values for completed suicide reached 3.15 in children and 3.11 in adolescents, however, the IC for the total population was 1.95.
The presence of fatal cases without previous anxiety/depression reported, the positive dechallenge in ideation, attempt suicidal or self injurious behaviours and the positive rechallenge in few cases, support the urgent need to implement well-designed epidemiologic studies that can lead to the quantification of the suicide/suicide attempt risk level among children using montelukast.”
Side Effects of Leukotriene Receptor Antagonists in Asthmatic Children
Semiha Bahceci Erdem, Hikmet Tekin Nacaroglu, Canan Sule Unsal Karkiner, Ilker Gunay, Demet Can
Iranian Journal of Pediatrics: Vol.25, issue 5; e3313, October 2015
https://brieflands.com/articles/ijp-3313.html
1024 cases from among the 13857 with early wheezing and asthma followed up at Dr Behcet Uz Children’s Hospital, Allergy Department during 2008-2013 who met the inclusion criteria were included in this study. Inclusion criteria for the study were 1) existence of early wheezing or asthma, 2) categorization of episodic wheezing or mild persistent asthma, 3) only LTRAs use as an anti-inflammatory therapy, 4) unavailability of determined findings before the use of drugs and disappearance of them after discontinuation of drugs; observation of the same side effect with the reuse of them, 5) no use of any other drugs even bronchodilator, when findings were observed…. While hyperactivity was the most frequently seen psychiatric side effect, abdominal pain was most common as non-psychiatric side effect in our study. Another interesting feature of our study was that convulsion related with leukotriene receptor antagonists was observed in 2 of our patients even though leukotriene receptor antagonist related convulsion is rarely reported…. in our study more than half of the cases were in preschool age, neuropsychiatric manifestations were observed…. a high (4%) rate of side-effects was observed…. The most commonly observed were psychiatric problems…. Patients should be informed in this respect and they should be assessed at certain intervals following the initiation of the treatment with leukotriene receptor antagonists. Moreover, comprehensive studies for determining the risk factors for leukotriene receptor antagonists related adverse drug reactions should be carried out.
Montelukast-Induced Adverse Drug Reactions: A Review of Case Reports in the Literature
Gioacchino Calapai, Marco Casciaro, Marco Miroddi, Fabrizio Calapai, Michele Navarra, Sebastiano Gangemi
Pharmacology 2014;94:60-70
https://karger.com/pha/article/94/1-2/60/289195/Montelukast-Induced-Adverse-Drug-Reactions-A
“Sleep disturbances, including nightmares, have not been described in clinical trials on montelukast, while several cases have been reported in post-marketing surveillance. Cereza et al. [46] reviewed 24 cases who reported nightmares, both in adults (n = 7) and in children (n = 17). Among them, 14 patients had other concomitant psychiatric symptoms, and in all these cases the suspect medication was montelukast. All these symptoms are, however, included in the SPC of montelukast [46]. Four children, aged between 1 and 5 years, developed sleep disorders (i.e. insomnia, somnolence and night terrors) as well as behavioural and mood disorders while receiving montelukast [47]. None of them had formerly reported psychiatric disorders. Three of the 4 children expressed suicidal ideation. After montelukast withdrawal, all these symptoms disappeared [47]. A 9-year-old boy on montelukast therapy for several years experienced neuropsychiatric events consisting of sleepwalking, sleep disturbance, bruxism and anxiety worsened by stressful events [48]. Kocyigit et al. [49] reported the case of a 13-year-old patient who had visual hallucinations after starting a therapy with montelukast, which disappeared within 48 h after the cessation of drug intake. Alkhuja et al. [50] observed the case of a 16-year-old female who received montelukast for asthma. In the nights following the therapy start, the patient’s mother reported daily parasomnias in the form of sleep talking and sleepwalking. Montelukast was discontinued which resulted in a disappearance of the parasomnias. After re-challenge, the parasomnias appeared again. Once montelukast was definitely discontinued, the parasomnias were never reported again [50]. In these articles, both adults and children seem to develop psychiatric symptoms after the intake of the medication. In every patient, the symptomatology seems to become less with the discontinuation of the therapy with montelukast within few hours. Although anti-leucotrienes are safe drugs, these symptoms have to be monitored especially in children.”
Alice in Wonderland syndrome: A rare neurological manifestation with microscopy in a 6-year-old child
Anne Weissenstein, Elisabeth Luchter, and M.A. Stefan Bittmann
J Pediatr Neurosci. Sep-Dec 2014
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4302569/
Cases of AIWS with the use of montelukast, a mast cell stabilizer, are described.
Institute for Safe Medication Practices Special Report on Children – QuarterWatch – January 2014
https://www.ismp.org/sites/default/files/attachments/2018-01/2013Q1-KidsSpecial.pdf
“Suspect Drugs Psychiatric disorders were reported for 25% or more of all adverse events for 10 of the 15 drugs on the QuarterWatch ranking of the most frequent suspects. The total included two drugs without intended psychotropic effects, montelukast (SINGULAIR for asthma and allergies) and isotretinoin (CLARAVIS for acne).” “Psychiatric adverse drug events, notably suicidal behaviors, represent the major adverse effects reported in children under age 18. Dechallenge—or careful, tapered discontinuation—is often a viable strategy for testing whether a drug is responsible for changes in behavior or mood.”
Neuropsychiatric disorders associated with montelukast
Burgos Pimentel, Montoro de Francisco, Tavakov, A., Fonseca Avendano, J., De Vicente Jimenez, Chivato Perez, Mateos Galvan, JM.
Poster session 1197, Asthma and allergy: how to improve treatment and patient education
Allergy (2014) 69 (Suppl. 99), 326–453, p. 439
https://onlinelibrary-wiley-com.ezproxy.uws.edu.au/doi/pdf/10.1111/all.12477
Background: Montelukast is a potent and selective antagonist of the cysteinyl leukotriene (Cys-LT1) receptor. Indicated as an adjunct treatment for the control of bronchial asthma; provides relief in allergic rhinitis. Rash, diarrheal evacuations, and neuropsychiatric disorders related to montelukast use have been observed after its commercialization, their incidence is low, but is not specified in the summary of product characteristics of this drug. These side effects are clinically relevant and might require psychiatric treatment or drug withdrawal.
Method: We present 2 childs: a female, 9 years old, After 6 months using montelukast presents nervousness, visual hallucinations (bugs), yielding gradually after 30 days of stopping the drug, and a male 3 years old, Starts montelukast 4 mg to asthma control, after 3 days presents nervousness, aggression, insomnia, nightmares, hallucinations (speech alone), generalised
rash, diarrheal evacuations, after 15 days therapy is discontinued and the picture refers gradually in 1 week. Background of loratadine and cetirizine nervousness. As well a male, 49 years old who has trouble sleeping and frequent nightmares, after a week starting montelukast, the patient removes the drug. Insomnia and nightmares wear off gradually over the next 5 days. No
personal or family history of psychiatric disorders any of the patients.
Results: In order to evaluate a possible causal relationship between montelukast and the neuropsychiatric disorders, and in the second child generalised rash, diarrheal evacuations and montelukast we used a modified Karch-Lasagna algorithm. In all cases, a causal relationship was qualified as probable.
Conclusion: In literature, all cases of nervousness, aggression, hallucinations and abnormal dreams, have been observed in a population-aged below 18. A health professional should pay a
special attention to these side effects, especially when it comes to a pediatric population.
Effect of Concomitant use of Montelukast and Efavirenz on Neuropsychiatric Adverse Events
Ibarra-Barrueta O, Palacios-Zabalza I, Mora-Atorrasagasti O, Mayo-Suarez J.
The Annals of Pharmacotherapy, 2014 Jan;48(1):145-8.
https://pubmed.ncbi.nlm.nih.gov/24259633/
“In November 2011, montelukast was started for asthma and shortly thereafter neuropsychiatric symptoms appeared, consisting of disturbed sleep, vivid dreams, irritability, confusion, and concentration difficulties. In January 2012, 2 months after the introduction of montelukast, she continued to report unbearable symptoms without any improvement; so, montelukast was withdrawn and the psychiatric symptoms completely disappeared.”
Hallucination development with montelukast in a child with asthma: case presentation
Aysen Kocyigit, Banu Gulcan Oksuz, Fulya Yarar, Funda Uzun, Mahir Igde, Ismail Islek
Iranian Journal of Asthma, Allergy and Immunology, 2013 Aug 28;12(4):397-9.
https://pubmed.ncbi.nlm.nih.gov/23996717/
“Leukotriene receptor antagonists(montelukast) have been used for many years in the treatment of asthma both acute and chronic stages. They are accepted commonly as safe but mostly possible side effects are ignored. However, montelukast also could lead to important adverse reactions like hallucinations. In literature only 2 reports have been found about hallucinations with it. One is a study which reports 3 patients from 48 children and the other is a 29 year-old case report. In our case, psychiatric adverse reactions of montelukast,especially hallucinations are reported similarly. We are presenting a child who had visual hallucinations after starting to use montekulast and after stopping the medicine these complaints disappeared in 48 hours. Although it is a safe drug, it should not be forgotten that it has psychiatric side effects which may be missed easily especially in children.”
Psychiatric disorders associated with montelukast: data from the National Pharmacovigilance Database
Marchand MS, Jonville-Béra AP, Autret-Leca E
Association française des centres régionaux de pharmacovigilance. Archives de Pediatrie 2013 Mar;20(3):269-73.
https://www.ncbi.nlm.nih.gov/pubmed/23375423
“Montelukast (Singulair(®)) has been the subject of post-marketing warnings about psychiatric events occurring that had not been identified duringclinical trials. The objective of this study was to take stock of the adverse events (AEs) related to montelukast reported in France. Cases of psychiatric disorders reported to regional pharmacovigilance centers (CRPV) and the literature data were analyzed. The 56psychiatric AEs account for 20% of all AEs reported in the montelukast CRPV: essentially sleep disorders, behavioral disorders and depression. This risk is also found in pharmacovigilance databases in other countries, especially in the North American database, which recorded a significant number of cases of “suicidality”, including suicidal ideation, suicide attempts, and suicides. Analysis of clinical efficacy studies have failed to confirm these AEs. The potential severity of these events prompts physicians to seek the existence of psychiatric disorders before prescribing the drug and to carefully monitor the occurrence of AEs during treatment.”
Case Report – Sleeptalking! Sleepwalking! Side Effects of Montelukast
Samer Alkhuja, Natalya Gazizov, and Mary Ellen Alexander
Case Reports in Pulmonology, Article ID 813786, 3 pages, 2013.
https://doi.org/10.1155/2013/813786
“A 16-year-old Caucasian female presented to the pulmonary clinic for a followup on her asthma. Due to the worsening of allergy-related symptoms, therapy with montelukast 10 mg daily was started and resulted in good relief of the patient’s symptoms. In the nights following initiating therapy with montelukast, the patient’s mother reported daily parasomnias in the form of sleeptalking and sleepwalking. Montelukast was discontinued, and that resulted in absence of the parasomnias. In a second attempt montelukast was reinstituted to control the patient’s symptoms. Parasomnias were immediately reported after resuming therapy. Montelukast was then discontinued indefinitely. Our patient has never had any history of parasomnias, and since the discontinuation of montelukast, parasomnias were never reported again. Parasomnias in the form of sleeptalking or sleepwalking were not previously reported as adverse effects of montelukast.”
A Case of Alice-In-Wonderland Syndrome Probably Associated with the use of Montelukast (article in Spanish)
Bernal Vañó E & López Andrés N.
Anales de Pediatria (Barcelona). February 2013
https://www.ncbi.nlm.nih.gov/pubmed/22857942
Delayed Onset of Neuropsychiatric Effects Associated with Montelukast
Byrne F, Oluwole B, Whyte V, Fahy S, McGuinness D
Irish Journal of Psychological Medicine, 2012 Jan;29(2):125-127.
https://www.ncbi.nlm.nih.gov/pubmed/30199961
“Montelukast (a leukotriene receptor antagonist) is a commonly prescribed medication used in the management of asthma in both children and adults. It has
been associated with a possible increased risk of various neuropsychiatric events in post-marketing analyses of clinical trial data and surveillance studies. When
establishing a link between a medication and side effects, it is usual to establish and enquire whether there is a chronological relationship between the
commencement of the medication and the onset of the symptoms. We report a case where a number of unusual neuropsychiatric events were reported several years
after commencement of montelukast in a young boy who may have a genetic predisposition and a likely psychological trigger. There was complete resolution of
these symptoms upon the withdrawal of montelukast.”
Nightmares Induced by Montelukast in Children and Adults
Gloria Cereza, Núria Garcia Doladé, Joan-Ramon Laporte
European Respiratory Journal 2012 40: 1574-1575;
https://erj.ersjournals.com/content/40/6/1574
“Up to December 2011, the Spanish System of Pharmacovigilance had gathered 24 reports of nightmares in patients (17 children, seven adults) treated with montelukast (table 1). Of the 24 patients, 15 were males and nine were females. 14 patients presented with other concomitant psychiatric symptoms: insomnia (n=5), nervousness (n=4), hallucinations (n=3), aggressiveness (n=2), irritability (n=2) and anxiety (n=1). Cases with aggressiveness were rated as serious. In all cases the only suspect medicine was montelukast. Six patients had taken other medicines concomitantly, although on a long-term basis. In 18 patients the nightmares appeared within the first day of exposure (n=11) or within the first week of treatment (n=7). Nightmares rapidly resolved after montelukast discontinuation in 21 cases. Alternative causes other than montelukast were excluded in most reports. Three patients were re-exposed to montelukast after the nightmares resolved, and in all three patients the nightmares reappeared.”
Neuropsychiatric reactions to montelukast
A Callero-Viera, S Infante, V Fuentes-Aparicio, L Zapatero, E Alonso-Lebrero
Journal of investigational allergology and clinicla immunology, 2012;22(6):452-3
https://www.jiaci.org/issues/vol22issue6/8-19.pdf
“We report on 4 children who presented neuropsychiatric side effects and mood disorders while receiving treatment with montelukast for asthma control. None of them had previous known
psychiatric disorders. The clinical features are shown in the Table. All the children presented extreme aggressive behavior. In fact, patient 3 was admitted to the child psychiatry unit because of aggressive behavior and autolytic ideation. At the time he was taking 10 mg daily of montelukast and salbutamol as rescue medication. In 2006, he had experienced a manic crisis attributed to prolonged treatment with inhaled corticosteroids, although he had also been taking montelukast 10 mg continuously for at least 2 years. Good tolerance to inhaled corticosteroids was confirmed and once montelukast was withdrawn all the psychiatric symptoms disappeared. In our experience, time to onset of symptoms from exposure to the drug ranges from 4 days to 3 months. Once montelukast was withdrawn all of the patients improved within 24 hours or several days at the most.”
Psychiatric adverse drug reactions reported during a 10-year period in the Swedish pediatric population
Maria Bygdell, Gertrud Brunlöf, Susanna M. Wallerstedt, Jenny M. Kindblom
Pharmacoepidemiology and drug safety, November 2011
https://onlinelibrary.wiley.com/doi/abs/10.1002/pds.2265
All spontaneously reported Individual Case Safety Reports (ICSRs) concerning children (<18 years old) and psychiatric adverse reactions assessed as at least possible, registered in the Swedish Drug Information System (SWEDIS) during the period 2001–2010, were extracted and characterized. Age and sex distribution and labeling/registration status were studied.
A total of 600 ICSRs concerning 744 psychiatric adverse reactions were identified and included in the analysis. Boys were overrepresented among included ICSRs (60.3% vs. 39.7%; p < .001). After exclusion of vaccines, the three most frequently suspected drugs were montelukast, centrally working sympathomimetic drugs, and inhaled glucocorticoids. Serious adverse reactions were reported more frequently for drugs used off-label than for drugs used according to the Swedish Physician’s Desk Reference. Aggressiveness was reported more frequently for boys than for girls as were suicidal conditions.
Psychiatric ADRs in the pediatric population have been reported for a wide range of reactions and drugs and display age and sex differences including a higher number of suicidal reactions in boys. An association was seen between serious reactions and off-label drug use. Further studies are needed to elucidate safety aspects of unlicensed drugs and drugs used off-label and whether there are differences in children’s susceptibility to develop ADRs.
Psychiatric and Behaviour-Related Adverse Events Occurring with Antiasthmatic Drugs Reported in the National Pharmacovigilance Network
F. Trotta, L Tartaglia, F. Ferrazin and C. Santuccio
Italian Medicines Agency, Pharmacovigilance Unit, Italy, INTERNATIONAL SOCIETY OF PHARMACOVIGILANCE ABSTRACTS, PP 231, 11th ISoP Annual Meeting, 26–28 October 2011
https://link.springer.com/article/10.2165/11596230-000000000-00000
Background:Antiasthmatic drugs (ADs) are frequently used in childhood; psychiatric and behaviour related adverse reactions have been reported especially after leukotriene inhibitors (LI).[1] An increased reporting of these reactions in children was observed in Europe.[2] Objective:To analyze and describe psychiatric and behaviour-related adverse events occurred with ADs and reported in the National Pharmacovigilance Network. Methods:Spontaneous reports occurring with ADs and suspected ADRs included in the SOCs Psychiatric Disorders and Nervous System Disorders were retrieved from January 2001 to June 2011.
Results: Overall, 712 reports of ADR with ADs use were identified; 170 (24%) were referred to the SOCs of interest. Considering different age groups, the 29% of cases were referred to children, while 45% and 26% were adults and elderly respectively. With regard to the drugs, in the 32% of cases the class of LI was involved (in all but one cases the drug involved was montelukast), followed by beta-2 agonists and association between beta-2 agonists and glucocorticoids (19% and 16% respectively).
Moreover, 51 cases referred to psychiatric and behaviour-related adverse events were reported in children. A very high percentage of paediatric reports (33 out of 51, 65%) with montelukast have been observed when compared with other ADs. The majority of the ADRs were not serious (70%), while the 19% were serious (for the remaining the seriousness was undefined). There is not a higher frequency of serious reports with LI.
A total of 241 ADRs with ADs were retrieved within the SOCs of interest. In particular, 88 out of 241 ADRs occurred with LI (57; 65% in children) while the remaining 153 ADRs with other ADs (only 25; 16% in children). The PTs more frequently reported in children following montelukast were insomnia, nightmares, headache, hyperactivity; the PTs more frequently reported in children following other ADs were agitation, insomnia, tremor, restlessness. The AD reports were analyzed also considering specific SMQs resulting in 55 events: Depression, excluding suicide and self injury (16 events), Hostility/aggression (27 events), Psychosis and psychotic disorders (10 events), Suicide/self-injury (2 events). The 51% of these SMQ events were referred to montelukast (28 out of 55), 22 of which (78%) occurred in children.
Conclusion:A high frequency of psychiatric and behaviour-related adverse events with montelukast, particularly in children, was high-lighted. Although some psychiatric ADRs are included in the montelukast product information, it is necessary to add more evidence to improve the appropriateness of use in children.
Association Between Leukotriene-Modifying Agents and Suicide: What is the Evidence?
Schumock GT1 , Lee TA, Joo MJ, Valuck RJ, Stayner LT, Gibbons RD.
Drug Safety. 2011 Jul 1;34(7):533-44.
https://www.ncbi.nlm.nih.gov/pubmed/21663330
“From 1998 to 2009 there were 838 suicide-related adverse events associated with leukotrienes reported to the FDA, of which all but five involved montelukast. Nearly all cases were reported in 2008 and 2009 (96.1%) after the FDA warnings. LTMAs are approved for use in asthma and allergic rhinitis, and are effective drugs. Both of these diseases are also associated with suicide, making confirmation of the association more difficult. Given the lack of good evidence, we recommend that a large observational cohort or case-control study be conducted to quantify the association between LTMAs and suicide. Until then, when prescribing LTMAs, clinicians should consider the potential for suicide and monitor patients who may be at elevated risk carefully for suicidal ideation or psychiatric symptoms associated with suicidal behaviour.”
Adverse Effects Associated with Leukotriene Antagonist Therapy
D. P. Gadde, P. S. Creticos, D. E. Beakes, P. L. Dauby, L. A. Grooms, D. Abeel, J. Gadde
Adverse effects associated with leukotriene antagonist therapy. Journal of Allergy and Clinical Immunology, 125(2), AB68. 2010
https://www.jacionline.org/article/S0091-6749(09)02064-8/fulltext
“Leukotriene receptor antagonists (LTRa) have become a mainstay in the treatment of allergic rhinitis/asthma. Recent reports have cited the potential neuropsychiatric disturbances observed in patients (pts) receiving this medicine [FDA healthcare professional update; June, 2009]. In a metropolitan private practice, we identify 20 pts with adverse effects that correlate with use of montelukast (MK). METHODS: We reviewed 2700 current pts in this practice and captured the self-reported adverse effects related to usage of MK. The demographic characteristics and descriptive statistics were tabulated for this patient population. RESULTS: Of these 2700 pts, 814(30%) used MK during the period of observation(July 2006-August 2009). Of the 20 pts [male:11; female:9; age:3-62 years(mean:16 yr; median:8 yr)] that self-reported adverse effects on MK, treatment duration ranged from 0.5 wks-8 years [mn:14 months(mo); med:7mo](<1 mo:3 pts;1-3mo:5pts;3-6mo:1pt;6-12mo:6pts; >1 year:5pts). Neuropsychiatric disturbances described were: a]behavior disturbances: aggression (6) anger (5) agitation (1) hyperactivity (4) emotional lability (6) misbehaving (1); b]sleep disturbances: insomnia (5) hallucinations (1) vivid dreams: (1) night terrors (1) screaming at night (1); c] anxiety (4); d] depression: (2); e] sense of doom: (1). Seventeen of 20 pts had complete resolution of adverse events [< 1 week: 7 pts; <1 mo: 7 pts; 1-3 mo: 3 pts], 2 pts report improvement, and 1 pt still reports night terrors. CONCLUSION: This report notes the association between use of a LTRa and onset of new neuropsychiatric disturbances. This data emphasizes the need for post-marketing surveillance in pts placed on this class of drug and the need to carefully monitor neuropsychiatric outcomes in a systematic manner.”
Montelukast and psychiatric disorders in children
Wallerstedt SM, Brunlöf G, Sundström A, Eriksson AL.
Pharmacoepidemiol Drug Safety 2009 Sep;18(9):858-64.
https://pubmed.ncbi.nlm.nih.gov/19551697/
“We analyzed all reports of psychiatric disorders during treatment with montelukast in children (<18 years) in the Swedish ADR database SWEDIS (1998-2007) … Psychiatric ADRs can occur during montelukast treatment in children, indicating that attention to this is essential. Further studies are needed to establish the magnitude of the problem. The fact that the patients (except for one) did not suffer from depressive symptoms before they started montelukast, the short latency, and recovery after withdrawal of the drug all strengthen our hypothesis that depressive symptoms are an ADR related to the use of montelukast. According to the Marketing Authorisation Holder of montelukast, depression will be added to the product information.”
QuarterWatch: 2008 Quarter 2
Institute for Safe Medication Practices Special Report on Children – January 2009
https://www.ismp.org/sites/default/files/attachments/2018-01/2008Q2.pdf
“Montelukast accounted for more possible cases of depression/suicidal behavior, hostility/aggression and psychosis than any other drug taken for any purpose in the second quarter … Hundreds of doctors, parents and patients reported possible psychiatric side effects of montelukast, once informed of a possible connection. A handful of cases prior to March 2008 were credible enough for the manufacturer, Merck & Co., to include them in the product information for physicians, and to trigger an independent FDA safety review, without either indicating it had confirmed a causal relationship. The mere public notice that such a review had begun, together with the addition of suicide to the product label, was enough to trigger hundreds of additional case reports. For an event to be reported in a voluntary system, a linked series of events has to happen: 1) it has to occur; 2) it has to be observed in credible detail; 3) a connection to the drug has to be suspected, and 4) the observer must elect to report it. The case of montelukast illustrates what happens when one link in this chain—a connection to the drug—is largely missing, and what occurs when healthcare professionals and consumers are informed of a possible connection. In the past, drug manufacturers have sought to discount spikes in adverse event case reporting when connected to publicity as “stimulated” reporting, as if the cases were somehow less valid than other reports. We believe the opposite: without patients and doctors getting adequate information about possible drug adverse events, the injuries caused by drug therapy will be substantially underreported in any kind of monitoring system. Rather than discounting these events, such spikes are evidence of the system beginning to correct undercounting that routinely occurs … After the FDA warning, psychiatric side effects accounted for 96% of all types of adverse events cases reported; prior to the warning the warning psychiatric side effects accounted for 4% of a small number of cases.”
Montelukast and Worsening of Hallucinations in Paranoid Schizophrenia
Anandan, N & Ibitoye, Francis. (2008).
Psychiatric Bulletin. (2008) 32. 276-276.
“We thought it possible that montelukast was aggravating the somatic and visual hallucinations and the medication was stopped. After 2 days the new symptoms subsided completely. Though some of the previously present psychotic symptoms were still there, the patient was less agitated than when on montelukast.”
Effect of montelukast on bacterial sinusitis in allergic mice
Annals of Allergy Asthma and Immunology, 2006 Sep;97(3):329-35.
Paneez Khoury, Fuad M Baroody, James J Klemens, Kenneth Thompson, Robert M Naclerio
https://pubmed.ncbi.nlm.nih.gov/17042138/
Background: In mice, allergic rhinitis augments the infectious and inflammatory response to Streptococcus pneumoniae-induced sinusitis.
Objective: To investigate the effects of cysteinyl leukotriene antagonism on the severity of bacterial infection.
Methods: We performed 3 parallel, placebo-controlled experiments. In the first, mice were ovalbumin sensitized and ovalbumin challenged to show the effects of montelukast on the allergic inflammation; in the second, we evaluated the effect of montelukast on S. pneumoniae infection; in the third, we used mice that were both allergic and infected. Montelukast was given starting 2 days after sensitization until the day before euthanasia. One day after drug treatment began, the mice were inoculated intranasally with S. pneumoniae in the infected groups. Nasal hypersensitivity was measured with histamine challenges before the first sensitization and on the day before euthanasia. On the fifth day after infection, mice were euthanized, nasal lavage was performed, bacteria were cultured, and inflammatory cells in the sinuses were quantified.
Results: Mice that were infected only tended toward having increased bacterial counts from nasal lavage in the montelukast-treated group. The mice that were allergic and infected experienced significantly higher bacterial counts (P < .05). All 3 montelukast treatment groups had significantly decreased eosinophil counts as well as T-lymphocyte counts.
Conclusions: Montelukast reduces the manifestations of allergic rhinitis in mice. Surprisingly, montelukast led to an increase in bacterial growth in infected mice. This suggests an effect of the cysteinyl leukotrienes on the innate response to bacterial infection.
FDA Pharmacology Review Montelukast 1998
https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/020829s000_Singular_Pharma.pdf
Merck data showing Brain/Plasma ratio in rats one hour after taking 10mg/kg montelukast is 10 per cent (0.12/1.23). After 24 hours it is 971 per cent (0.68/0.07). While the concentration of montelukast decreased in all other tissues after 24 hours, in brain tissue it increased 467 per cent (p. 14).