All patients and doctors should receive the same warnings about montelukast’s side effects. But US patients and doctors get far stronger warnings than Australians.
In 2020, the US FDA also issued a Medication Guide (MG) [which must be provided to patients when dispensed]. MGs are issued when the FDA determines: “The drug has serious risk(s) (relative to benefits) of which patients should be made aware because information concerning the risk(s) could affect patients’ decision to use, or to continue to use, the product.”
Boxed warning, top first page for US doctors
https://www.organon.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf

Detailed information on page 5 for US doctors

Warning of some effects, middle page 3 for Australian doctors
https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-04106-3

Some extra effects listed on page 8 for Australian doctors

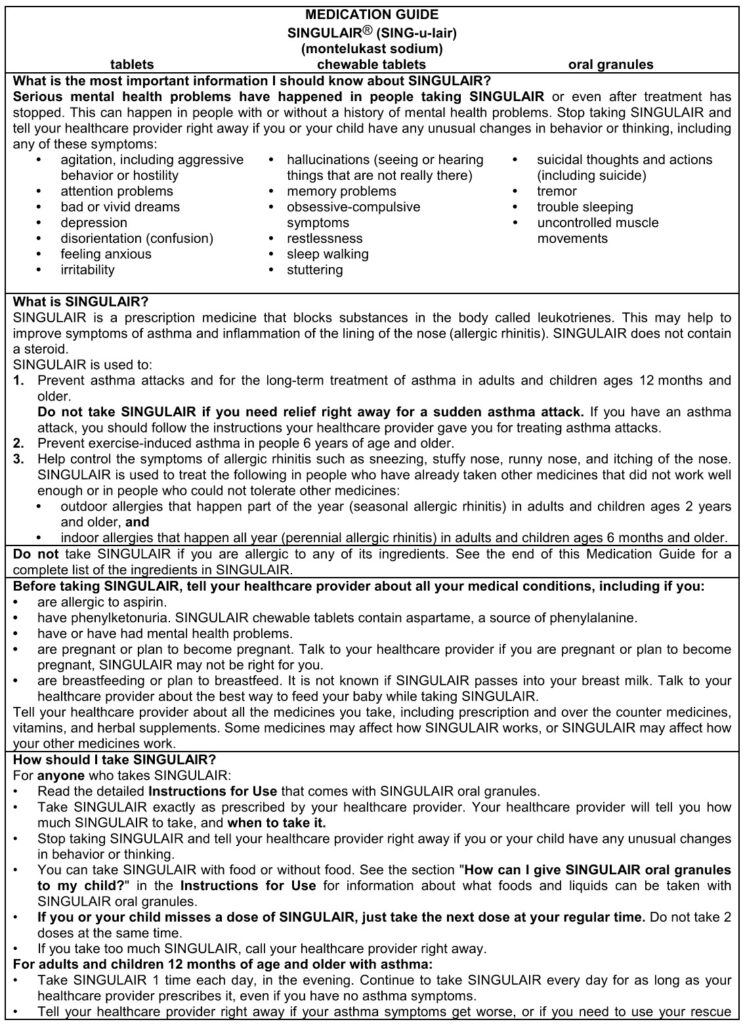
Warning, top first page for US patients
https://www.organon.com/product/usa/pi_circulars/s/singulair/singulair_mg.pdf
Warning middle page 3 for Australian patients
https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-CMI-04762-3&d=20220714172310101

Why different warnings for the same drug?
Singulair
Sign and share the petition to insist Australian consumers are adequately warned about the potential mental health side effects of this drug.